This article is regarded the h3o+ lewis structure and essential properties related to the h3o+ lewis structure. This will illustrate some crucial facts about the h3o+ lewis structure.
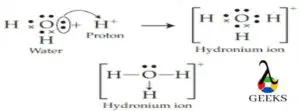
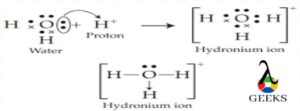
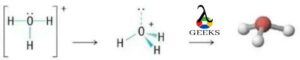
The h3o+ lewis structure is an important ion which is commonly called hydronium ions that are formed from water by protonation. It is a positive ion that is always produced by Arrhenius acids in a chemical reaction that losses proton in the reaction in its solution form.
The H+ ion and conjugate base are produced by the H3O+ Lewis structure in an aqueous solution. In general, all the trivalent oxygen cations are commonly called oxonium ions, so another name for hydronium ions is oxonium ions.
How to draw the H3o+ lewis structure?
The h3o+ lewis structure is important in chemistry and we will study it in acid-base chemistry and commonly considered acid. The total number of free electrons and bonded electron pairs in the atom is also represented by the Lewis structure.
The internal shells of a molecule are not taken into account by the Lewis structure, only the valence shell electrons are.
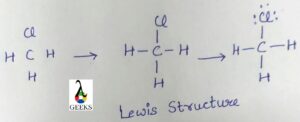
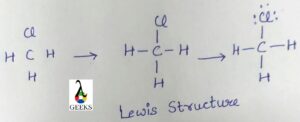
The h3o+ lewis structure is drawn by the following step:
Step:1 Find valence electrons in H3O+
For the h3o+ lewis structure study about the periodic table, we will calculate the valence electrons present in hydronium ion from the periodic table. Thus in H3O+, is total of 8 valence electrons are present.
Step:2 Find electronegative Atom
After calculating the valence electrons, we have to find the electronegative elements present in the centre when we make the lewis dot structure. In the H3o+ case, we have oxygen is in the centre atom due to the more electronegative atom, and hydrogen as the outside atom.
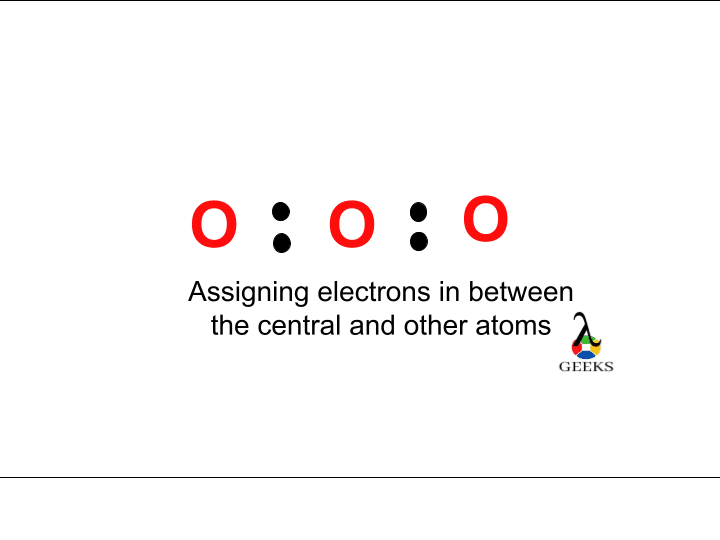
Step:3 Assign the valence electrons around each atom
It is possible to distribute the electrons around the central atom after knowing the electronegative atom and the valence electrons. Two electrons are put around it and make a chemical bond around it.
Step:4 Fullfill octet of each atom
The outside octet of atoms is completed after assigning the valence electrons around the central atom.
Step:5 Assign the remaining valence electrons to the central atom
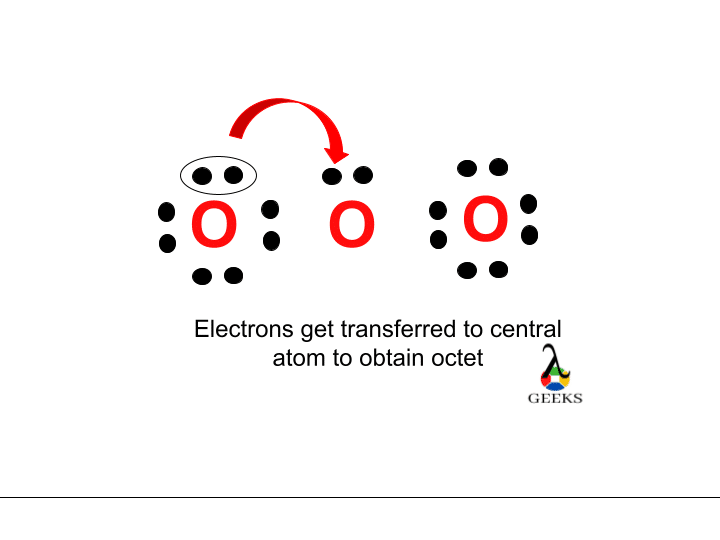
Creating double or triple bonds in an atom without an octet requires electrons to be moved between outer atoms and the central atom. We have lost a valence electron in H 3 O + as indicated by the + sign in the Lewis structure. Therefore, the H3O+ Lewis structure only has 8 valence electrons.
H3o+ lewis structure resonance
From the lewis structure of H3O+ we can see that it is isoelectronic with Ammonia molecules because the centre atom of both the molecules is electronegative such as O+ and N having the same number of electrons.
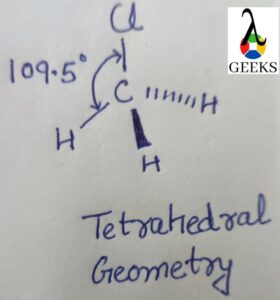
The H3O+ Molecule has a symmetric structure and trigonal pyramidal geometry with an angle of 113 degrees.
H3o+ lewis structure shape
Atoms are arranged in three dimensions in molecular geometry, and molecular geometry can be used to determine the physical and chemical characteristics of a molecule. The H3O+ ion is shaped like a trigonal pyramid. On the central oxygen atom of the hydronium ion, there are three O-H bonds and one pair of unpaired electrons.
As a result, four areas of electron density are formed around the main oxygen atom. The shape of the hydronium ion is trigonal pyramidal due to the uneven charge distribution surrounding the central oxygen atom. The H3O+ lewis structure has a pyramidal shape with three hydrogen atoms lying at the corners of oxygen and making a triangle.
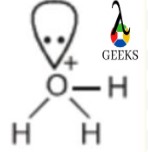
Thus the H3O+ shows trigonal pyramidal molecular geometry and pyramidal shape due to the presence of one lone pair at the oxygen atom. The H3o+ lewis structure has electronic geometry that is Tetrahedral.
H3o+ lewis structure formal charge
The formal charge on a molecule can be calculated by the following formula,
Formal Charge= Free atom consists of the valence electron – (the number of non-shared electrons – ½ shared electrons around the atom). The H 3 O + molecule is composed of three bonds and one lone pair with a +1 formal charge.
Hydrogen belongs to the 1st group in the periodic table and has one valence electron, in a similar way oxygen belongs to group 14th group in the periodic table and consists of six valence electrons.
F.C on 3H = VE- NE- ½(BE)
= 1 – 0 – ½ (2) = 0
F.C on O = 6 – 2 – ½ (6) = +1
Thus H3O+ having a formal charge is +1.
We are aware that the more stable the Lewis structure of a given molecule is, the lower its formal charge value must be. The most stable Lewis dot structure of the H3O+ ion is because the hydronium ion only has a +1 formal charge, the lowest one.
H3o+ lewis structure angle
The angle that the central atom forms with the bonded atom are referred to as the bond angle. Due to the attraction of electron density regions surrounding the central atoms, the Bond angle varies between molecules.
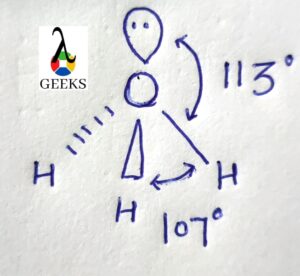
H3O+ ions have a bond angle of 113 degrees with four high electron density regions. The central atom in H3O+ is Oxygen which formed three O-H bonds and one lone pair of electrons. Thus the geometry of the H3O+ ion is trigonal Pyramidal with a 113-degree bond angle formed.
H3o+ lewis structure octet rule
The octet rule describes an atom’s natural tendency or desire to have 8 electrons on its valence shell through the loss, gain, or sharing of electrons. The atoms take on the electronic structure of the closest noble gas by gaining, losing, or sharing electrons.
All elements adhere to the octet rule, except for hydrogen and helium. The duplet rule applies to gases such as hydrogen and helium. In the case of H3o+(hydronium ion), the oxygen atom is charged with octets and the three hydrogen atoms are charged with duplets.
H3o+ lewis structure lone pairs
The oxygen atom has one lone pair and three single bonds connecting it to the hydrogen atoms, forming the Lewis structure H3O+. The oxygen atom has a positive charge because it exhibits fewer electrons in the Lewis structure of H3O+.
Thus, the Lewis structure of the hydrogen ion contains just one pair of electrons.
H3o+ valence electrons
The term “valence electrons” refers to the total number of electrons that are present on an atom’s outermost shell. In the formation of any chemical bond, only the valence electrons are involved. For such formation of the bond, they must either be redistributed or shared total or partial.
There are six electrons in the outermost shell of oxygen, and one electron in the outermost shell of hydrogen. Thus the total valence electrons in Hydronium ions is 8(6+1*3-1), due to their positive charge on it.
H3o+ hybridization
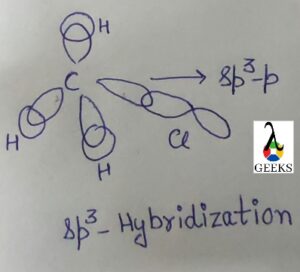
To, the process of hybridization combines atomic orbitals to create new hybrid orbitals for pairing electrons in chemical bonds. Due to its three O-H sigma bonds and one lone pair of electrons, the hydronium ion (H3O+) exhibits sp3 hybridization. Steric numbers can also be used to calculate hybridization.
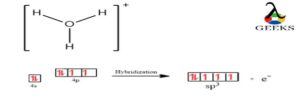
Steric Number= Number of atoms bonded to the central atom + Number of lone electrons pair in that atom.
In H3O+ ion has, thus the Number of Steric in Hydronium ion = 3 + 1= 4(sp3)
The presence of sp3 hybridization is indicated by the 4.
H3o+ solubility
An acid is a compound that, when dissolved, releases a proton(s) or H+, as per Arrhenius’ concept of acids. Now that an Arrhenius acid has released H+, the proton interacts with a water molecule to form a hydronium ion or H3O+ ion.
A solution of pure water and an acid increases hydrogen ion concentration. The hydroxide ion concentration must fall for [H3O+] [OH-] to remain constant. As a result, the solution is referred to as acidic because [H3O+] > [OH-]. The opposite is true if a base is added to pure water.
Is h3o soluble in water?
Yes, it produces oH– and H3o+ ions in the water and is soluble in water.
Why is H3O+ soluble in water?
Hydronium ion is categorized as or type of oxonium ion which consists of three ions in particular molecules. So, the production of Hydronium ions is from water molecules and water act as a base and Hydronium ion act as conjugate acid.
How is H3O+ soluble in water?
H+ ions are created in the solution when acid and water are combined. The hydronium ion (H3O+), which is formed when these ions combine with water molecules, cannot exist by itself.
H++ H2O → H3O+
Is H3o+ an electrolyte?
The solution would conduct electricity very poorly or not at all if H3O+ have been the stronger acid.
Why is H3O+ an electrolyte?
Its attraction to oppositely charged ions is so powerful that the hydrogen ion (H+) bonds to a molecule of water to create the hydronium ion (H3O+), denoting the absence of free hydrogen in water.
How is H3O+ an electrolyte?
It forms H3O+ when H2O gains an H+, Because they readily donate H+, strong acids are good examples of strong electrolytes because their dissociation in water is almost entirely complete.
Strong acid HCl dissociates in water, transferring H+ to H2O. The dissociation of 1 mole of a strong acid in water produces 1 mole of hydrogen ions and 1 mole of its conjugate base. The resulting solution essentially only contains H3O+ (a strong electrolyte) and Cl-.
Is H3o+ acidic or basic?
There are eight valence electrons in the Lewis structure of H3O+. We have lost a valence electron in H3O+ as indicated by the + sign in the Lewis structure. Therefore, the H3O+ Lewis structure only has 8 valence electrons. H3O+, which is classified as an acid.
Why is H3O+ acidic?
The hydronium ion (H3O+) is the designated Lewis acid in this instance, it only serves as the source of the proton that interacts with the Lewis base.
How is H3O+ acidic?
H+ attacks water solvent to form hydronium H3O, In reality: H+ + H2O gives H3O+, H+ protons & H3O+ Hydronium ion (H+ (aq) and H3O+ (aq)) considered the same. These terms are used interchangeably.
Is H3o+ a strong acid?
Dilute aqueous solutions contain only H3O+ as the strongest acid.
Why is H3O+ Strong acid?
Water becomes H3O+, an acid that is known as the conjugate acid of water when it acts as a base. Acids are substances that dissociate to give H3O+ ions. H3O+ should then be the strongest acid that is available since it doesn’t even require dissociation to function. In the presence of water, the hydronium ion becomes acidic.
This occurs when water molecules interact to form H3O+, which serves as a base in a chemical reaction and is a conjugate acid for water.
How is H3O+ strong acid?
The hydronium ion becomes acid when water act as the base, during the reaction of water molecules creation of H3O+ takes place which is a conjugate acid for water which behaves as a base in some chemical reaction.
Is h3o+ an arrhenius acid?
In an aqueous solution, an Arrhenius acid is a substance that ionizes to produce hydrogen ions (H+). Acids are chemical substances that contain ionizable hydrogen atoms. Ionizability only applies to hydrogen atoms that are a part of a highly polar covalent bond.
Why h3o+ an Arrhenius acid?
Thus, a material that disintegrates in water to create H+ ions is defined as water. It also meets the criteria for a substance that separates into OH- ions in water. This is the only Arrhenius amphoteric chemical because it is both an Arrhenius acid and a base.
How h3o+ an arrhenius acid?
An acid is a substance that raises the concentration of H+ or proton in an aqueous solution, To create the hydronium ion (H3O+), which is not a free-floating proton, the proton, or H+ ion, that is released coexists with the water molecule.
Is H3o+ polar or nonpolar?
H3O+ is a polar molecule because it has two lone pair electrons on top, which causes electron-electron repulsion.
Why H3o+ is polar or nonpolar?
Hydroxyl ions have an electronegativity of 3.44, while hydronium ions have an electronegativity of 2.20. Therefore, the difference in electronegativity is 1.24. 0.4 to 1.7% of the difference in electronegativity falls in this range. Thus, there will be a polar covalent bond in the O-H bond.
How H3o+ is polar or nonpolar?
It is the dipole moment of a molecule that determines its polarity. Dipole moments are calculated by dividing the charge amplitude by the distance between positive and negative charge centres.
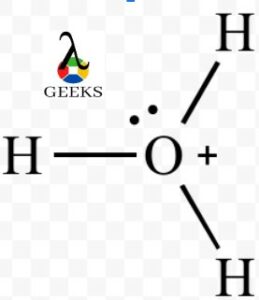
A dipole moment is created when the atoms surrounding the central atom are arranged asymmetrically. Around the oxygen, there are four asymmetrical regions which create a net dipole moment and make the hydronium ion polar.
Is H3o+ a lewis acid?
According to the Lewis structure of H3O+, the + sign represents a valence electron that has been lost. Acid-base chemistry depends heavily on the Lewis structure. H3O+, therefore, functions in chemistry as Lewis acid.
Why H3O+ is a lewis acid?
The conjugate acid of H2O is H3O+. In an aqueous solution, a proton is denoted by the symbol H3O+.
How H3O+ is a lewis acid?
A different proton structure would form in a non-aqueous solution. This demonstrates that H2O is amphoteric (can be an acid or a base) and has a deprotonated form (H3O+, or OH-) consisting of an equal mixture of H+ and OH- ions (OH-).
This will lead to the formation of strong acid in the aqueous solution.
Is H3o+ linear?
No, H3O+ is not linear, Since the O is linked to three hydrogen atoms and has a lone pair, giving the molecule H3O+ four-electron densities, the molecule is tetrahedral. The shape would be trigonal pyramidal because there is only one isolated pair.
Why is H3o+ linear?
The three hydrogen atoms that makeup oxygen are arranged in a triangle at its three corners, and one lone pair of oxygen’s electrons gives H3O+ its pyramidal shape is a solitary pair.
How is H3o+ linear?
Since theH3O+ Lewis structure contains an overall of 8 valence electrons, oxygen consisting three bonds with hydrogens and one lone pair on its own.
This causes the central atom of oxygen surrounded by four regions of electron density, giving the hydronium ions a tetrahedral structure despite their trigonal pyramidal shape.
Is H3o+ paramagnetic or diamagnetic?
Yes, H3o+ is a paramagnetic ion with positive signs and unpaired electrons are present in it and act as lewis acid.
H3o+ boiling point
Hydronium ion does not have its boiling and melting point because it exists in ionic form in the aqueous solution in form of H3O+ and OH- form. So that water has the boiling point, hydronium also shows the same boiling point.
H3o+ bond angle
It exhibits sp3 hybridization and has a bond angle of 109.5 degrees, according to the VSEPR theory. The precise bond angle, however, is 113 degrees because a positive charge and lone pair are present.
Three other atoms and one pair of electrons surround the central oxygen (O) atom of the H3O+ Lewis structure.
Is h3o+ amphoteric?
It is referred to as being amphoteric when a species can act as both a base and an acid, and as such, it must have the capacity to both accept and donate protons when required. We know that water is amphoteric, for example.
The protonated form of water is H3O+, so it can also behave as a strong acid. So, the answer is no, hydronium ion is not amphoteric.
Why is h3o+ not amphoteric?
Due to the amphoteric nature of water, H2O can function as a base by either acting as a proton donor or receiver and forming H3O+ and OH-. Thus Water acts as a base in an acidic medium and gives conjugate acid in the solution.
How is h3o+ not amphoteric?
In the presence of a base, a proton can be given to it to form the hydroxide ion, or a proton can be accepted from acid to form the hydronium ion (H3O+). An autoionization process produces OH ions and H3O+ ions from liquid water.
This isn’t amphoteric because it will result in the formation of acidic ions.
Is h3o+ a bronsted base?
No, H3O+ is bronsted acid in an aqueous solution, so that it losses H+ ion and gives water molecules. To be referred to as a “strong” conjugate acid, the conjugate acid of a base must want to lose a proton more than H3O+.
Why is h3o+ not bronsted base?
When an Arrhenius acid (a species that dissociates in water to form hydrogen ions, for example, HCl) is dissolved in water, H3O+ is created when water and acid combine. Thus the formation of bronsted lowry acid takes place.
Is h3o+ dative bond?
Yes, H3O+ did create a dative bond when its Lewis structure was being formed. During the formation of H3O+, one pair of O-atom lone pairs is donated to the open 1s-orbital of the H+ ion, forming an O-H covalent bond.
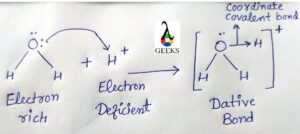
As a result, there are two O-H covalent bonds and one O-H co-ordinate bond in the H3O+ ion. After formation, the two O-H covalent bonds and the O-H coordinate bonds are identical and a dative bond exists in this ion.
Why has h3o+ formed a dative bond?
The positive ion in an Arrhenius acid solution is known as hydronium. It is made up of water and hydrogen ions. O-H is composed of two polar covalent bonds and one coordinate covalent bond.
When H+ and H2O combine to form the hydronium ion, H3O+, this bond is referred to as a coordinate covalent bond. For bonding electrons, oxygen provides all its valence electrons.
Is h3o+ a buffer?
No, the H3O+ lewis structure does not act as a buffer solution in the conjugate base in the buffer consumes the hydronium ion, turning it into the water and the conjugate base’s a weak acid when a strong acid (H3O+) is added to the buffer solution.
As a result, there are more weak acids present and fewer conjugate bases as well.
Is h3o hydrogen bonding?
Hydrogen bonds cannot be formed by the hydronium ion (H3O+). An intermolecular force called hydrogen bonding is present. There are three polar covalent bonds within a single H3O+ ion (between the oxygen atom and 3 hydrogen atoms).
Why has h3o+ formed a hydrogen bonding?
The hydrogen bonding force acts between molecules. Within a single ion of H3O+, there will be three covalent but polar bonds between the oxygen atom and each hydrogen atom. The positive charge will cover the entire ion.
How has h3o+ formed a hydrogen bonding?
A hydrogen bond can be created by any one of the three hydrogens joining the oxygen on a nearby water molecule. It is unlikely that the two positively charged ions will approach and form hydrogen bonds with other hydronium ions(H3O+), even though they would repel one another.
Is h3o+ a conjugate base?
Water acts as an acid when reacting with bases, releasing a proton to create its conjugate base, OH. Thus, OH is the conjugate base of water. A base is a proton acceptor in the Bronsted-Lowry concept.
It gains hydroxyl ions and behaves like a base to form conjugate acids H3O+, which is similar to how it acts when reacting with acid. Therefore, H3O+ is a conjugate acid.
Is h3o+ greater than oh-?
Yes, H3O+ which is present in an aqueous solution related to the ph is greater than ho- in that solution. Acidic and basic solutions, respectively, contain H3O+ and OH-. More H3O+ is present in an acidic solution.
The amount of hydronium ions (H3O+) in an acidic solution is greater than the number of hydroxide ions (OH-). The mixture would be neutral if the two concentrations were equal. The answer would be straightforward if [H3O+] is less than [OH-].
Why is h3O+ greater than Oh-?
In neutral aqueous solutions at 25 °C, H3O+= OH-. Hydronium ion(H3O+) will always be greater than hydroxyl ion(OH-) in an acidic solution, such as vinegar (acetic acid in water).
If the solution is basic, such as sodium hydroxide (NaOH) in water, the opposite is true. we may find it difficult to understand that acidity increases as pH decreases and vice versa because pH = -log[H3O+].
How is h3O+ greater than Oh-?
We are aware that the following equilibrium exists in an aqueous solution: 2H2O(l)⇋H3O+ +HO, The so-called “autoprotolysis,” or self-ionization of water.
Additionally, HO- stands in for the basic principle while H3O+ represents the acid principle.
Additionally, under normal circumstances,
Kw=[H3O+][HO−]=10−14
Kw=[H3O+][HO−]=10−14
And so in a NEUTRAL solution,
[H3O+]=[HO−]
but in an ACIDIC solution,
[H3O+]>[HO−].
According to standard conditions,
pH=−log10[H3O+], and pOH=−log10[HO−].
And under standard conditions, pH+pOH=14
Is h3o+ dipole moment?
The lone pair on the oxygen atom in the H3O+ molecule also contributes to the explanation for the polarity. H3O+ is a polar molecule because the net dipole has a non-zero value.
Why is h3O+ is showing a dipole moment?
Due to the presence of two lone pair electrons on top of the molecule, which causes electron-electron repulsion, H3O+ is a polar molecule.
As a result, the structure is bent or trigonal pyramidal which causes an uneven distribution of charge within the molecule.
How is h3O+ showing a dipole moment?
The electron clouds on atoms and the single pair of electrons surrounding the O atom will repel one another according to the Valence Shell Electron Pair Repulsion Theory (VSEPR Theory).
They will consequently be forced apart, giving the H3O+ molecule its trigonal pyramidal shape. Additionally, the oxygen in this molecule carries a positive charge, making it a cation with inherent polar properties.
Is h3o+ a reducing agent?
No, the Hydronium ion is act as an oxidizing agent because it absorbs electrons and gives the redox reaction.
Is h3o+ an oxidizing agent?
Other names for oxidizing agents include oxidants and oxidizers. Another way to think of an oxidizing agent is as a species that can transfer electronegative atoms, in particular oxygen, to a substrate.
The oxidising agent is that agent which steals the electrons from other reactants and makes the solution acidic. All metals that react with 1 M HCl in which H3O+ ions are the active oxidizing agent produce a metal ion in the solution.
H3o+ is electrophile or nucleophile
H3O+ (Hydronium) lacks a vacant orbital in the valence shell, making it impossible for it to gain electrons. However, H3O+ continues to act as an electrophile because it dissociates into H2O and H+.
H+ acts as an electrophile because it is capable of acquiring electron pairs.
Why does H3O+ is behaving as an electrophile?
H3O+ has a single pair of electrons, but because it also carries a positive charge, it is unable to donate that pair. As a result, it has no nucleophilic effect.
How does H3O+ is behaving as an electrophile?
H3O is not an electrophile because it has a lone pair of electrons available for donation among the others, indicating that it is not electron-deficient. Due to the electrons that are present in the 2p orbitals of O, I anticipated that H3O+ would be a nucleophile.
Due to a lack of open orbitals in its valence shell, H3O+ cannot gain electrons. H+ can gain an electron pair and thereby act as the electrophile when H3O+ dissociates into H2O and H+.
Is h3o+ an electrolyte?
H+ ions always form in solution when a strong electrolyte ionizes (breaks up). Only one of the strongest acids, H3O+, can be found in high concentrations in dilute aqueous solutions.
When [H3O+] changes, [OH-] changes in the opposite direction and vice versa. Lower [OH-] and higher [H3O+] Lower [H3O+] and higher [OH-]
[H3O+] = 1•10-7 M for pure water.
Is h3o+ a free radical?
Yes, The radical H3O+ readily disintegrates into a water molecule and a hydrogen atom and is only kinetically stable in the gas phase.
Why does H3O+ is behaving as a free radical?
When H3O is solvated by a single water molecule, the majority of its radical properties are maintained; however, two water molecules shift the majority of the spin density into the solvent.
How does H3O+ is behaving as a free radical?
Since it is only kinetically stable and easily breaks down into a water molecule and a hydrogen atom in the gas phase, the radical H3O has a localized spin density on its hydrogen end.
Is h3o+ hydrolysis?
A hydrolysis reaction is the breakdown of chemical bonds caused by the addition of water or a base that provides the hydroxyl ion (OH). Two new bonds are formed with either the hydroxyl (OH) or hydrogen (H) of the water molecule attached to each one after a chemical bond is broken.
Hydrolysis is the process of an ion reacting with water to produce H3O+ or OH-.
Is h3o+ monatomic or polyatomic?
Polyatomic ions, which have more than two atoms and more than two charges (positive for cations and negative for anions), are made up of more than two atoms. When naming any species, the positive ion, or cation, is always mentioned first.
Acids release hydrogen ions into the water, which combine to form the polyatomic ion H3O+, which is why acids and the H3O+ ion are related.
Why is H3O+ are polyatomic?
More than two atoms with either a positive or negative charge make up a polyatomic ion. An H3O+ polyatomic ion, which consists of three hydrogen atoms and one oxygen atom, has been presented to us.
How is H3O+ are polyatomic?
This ion is produced whenever an acid dissolves in water. The acid releases the hydrogen ion, which combines with the water molecule to form H3O+.
It goes like this: HCl(aq)+H2OH3O+(aq)+Cl (aq)
As the water-based hydrogen oxide protonates with the hydrogen released by acids, the oxide ion acquires the name oxonium, and the H3O+ion with hydrogen is known as the hydronium ion. So, H3O+ion is also known as hydronium ion which is a polyatomic ion.
Is h2o or h3o more acidic?
H3O+ is the conjugate acid of H2O. The conjugate acid will invariably be more potent than the conjugate base. The first proton will always be easier to remove than the second, regardless of the proton donor.
Why is H3O+ are acidic?
The most powerful acid that can coexist in an aqueous environment is H3O+ (H+). As a result, the reactants H2O, that act as the base, will benefit from the equilibrium in this system.
Water (H2O) can act as amphoteric till then it undergoes any reaction that makes it either an acid or a base depending on what it is reacting with. However, the purest water is always neutral because it has a pH of 7 and has an equal number of H+ and OH- ions (neither acidic nor basic).
How is H3O+ acidic?
H3O+ is the strongest acid that can exist in water in an aqueous environment with HO-. As a result, the equilibrium that exists in the system will be useful to the reactants, H2O which is amphoteric.
Is h3o neutral?
No, Acids are substances that raise the concentration of H3O+ in an aqueous system. This should be written H3O+ because OH- and hydrogen ions are a better representation.
Why is H3O neutral?
Water becomes H3O+, an acid that is known as the conjugate acid of water when it acts as a base. The most potent acid in an aqueous solution is H3O+. Since there is too much H3O+ in an acidic solution, OH- decreases.
Because there is too much OH- in a basic solution, H3O+ decreases.
Is h3o stable or unstable?
H3O+ is a stable ion in an aqueous solution, in contrast to the nonionized form of a strong acid, but it will interact with bases to form weak acid water. As a result, an equilibrium is reached in pure water when an equal amount of the strong base OH- and H3O+ are formed and then react to reform water.
Why is H3O+ Stable?
At room temperature, all aqueous solutions have base (OH-) and acid (H+) effective ions. Lower pH = less acid. More base equals a higher pH. The inverse of the H+ ion concentration in powers of ten yields the pH neutral value of 7.
How is H3O+ stable?
A proton with an empty 1s orbital that has room for up to two electrons is known as an H+ ion. It wants an electron so desperately that it sinks to the centre of the earth. In fact, with the positive charge now evenly distributed among all of the H atoms, the entire molecule is H3O+.
One of the more stable ions is H3O+. The first water molecule, which is crucial for comprehending the chemistry of the world’s oceans, will attract other water molecules to itself.
Is h3o+ symmetrical or asymmetrical?
H3O+ is asymmetrical due to given that H3O+ has a tetrahedral structure and eight total valence electrons, each of the three H atoms and the O must be connected by a bond, leaving the O with a single pair of electrons.
Why is H3O+ is Asymmetrical?
H3O+ has 3 bonds and 1 lone pair, but because of this, its shape is trigonal planar. When examining a molecule, be sure to decide whether you are searching for shape or electron arrangement Polar molecules with asymmetries.
How is H3O+ is asymmetrical?
The Lewis structure has eight electrons, and when it is drawn, the oxygen is connected to the other two atoms by three bonds and one lone pair. Tetrahedral molecular geometry results from the presence of 4 electron domains.
Due to one lone pair present on the oxygen atom, the molecules obtained a trigonal pyramidal shape. Four regions of electron density give H3O+ its tetrahedral electron arrangement which is asymmetrical.
Is h3o+ planar?
Due to asymmetry in the molecules, it shows trigonal geometry, so it is not a planar molecule and exhibits a tetrahedral structure with sp3 hybridization.
Why is H3O+ not planar?
Three hydrogen atoms and one oxygen atom make up the trigonal pyramidal geometry of the hydronium ion. The oxygen has this shape because of a single pair of electrons on it. The atoms’ 113-degree bond angle is measured between them.
Is h3o+ protic?
Having a hydrogen atom bound to oxygen in HO-, nitrogen in NH2-, or fluoride makes a solvent protic in HF solvents. A potent intermolecular force can take place in protic solvents. Additionally, protons (H+) can be obtained from these O-H bonds. Thus H3O+ is protic and exhibits an acid and polar nature.
Is h3o+ trigonal pyramidal?
Yes, H3O+ is having Trigonal pyramidal structure or shape with tetrahedral geometry which consists of sp3 hybridization with steric number 4.
Why is H3O+ trigonal pyramidal?
The shape of H3O+ is pyramidal due to the oxygen atom is joined to three hydrogen atoms. The oxygen atom also has one single pair of electrons. A lone pair of electrons is an electron pair that resides in the atom’s orbital but is not directly involved in the bonding.
How is H3O+ Pyramidal?
There are no electrons in the hydrogen ion. Two lone pairs of electrons are present in the oxygen atom of the H3O+ lewis structure. The coordinate covalent bond is created as a result of the oxygen atom sharing one of its single pairs.
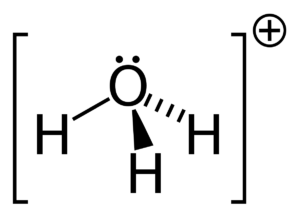
As a result, the hydronium ion contains the coordinate bond, following is the reaction: H2O+H+ → H3O+
Thus H3O+ is trigonal pyramidal in shape.
Conclusion
The structure, bonds, and hybridization of hydronium ions are the main topics of this article. It also explains that hydronium ions have a variety of uses and give immense deep knowledge regarding H3O+ lewis structure and properties.
Also Read: