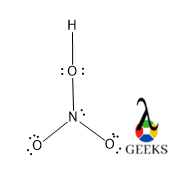
This article should contain the HNO3 lewis structure and its different important facts. Let’s discuss the HNO3 lewis structure.
In the HNO3 lewis structure, the molecule is consist of N, H, and three O atoms. All the atoms in the HNO3 lewis structure make a covalent bond. The N is the HNO3 lewis structure is sp2 hybridized while O is sp3 hybridized. There is one -OH group and two ketonic groups are present. Due to the presence of that -the OH group makes the molecule acidic. The electronegativity of O and N is so high that make that H is more acidic.
HNO3 or Nitric acid is one of the strongest inorganic acids. The aqua regia is made from this acid. The structure of the HNO3 is different because here two central are different hybridization. Around the N it is trigonal planar and around the O it is tetrahedral.
Some important facts about HNO3
The physical state of nitric acid is liquid. The color of the nitric acid is colorless but when it is stored for a long time its color changed to yellowish due to the decomposition of oxide of nitrogen. The boiling point and the melting point of Nitric acid are 356 K and 231 K respectively. The molar mass of this acid is 63.012 g/mol. The odor of nitric acid is acidic and very suffocating. The density of this acid is 1.51 g/cm3. Fuming nitric acid has vapor pressure and the value is 48 mmHg at 200C.
It can be produced by the reaction of water and nitrogen dioxide.
4 NO2 + 2 H2O → 2 HNO3 + NO + NO2 + H2O
The net reaction is,
3 NO2 + H2O → 2 HNO3 + NO
Nitrogen dioxide bubble goes through the hydrogen peroxide improving the yield of the product.
2 NO2 + H2O2 → 2 HNO3
In the laboratory, nitric acid can be prepared by the thermal decomposition of copper nitrate to produce nitrogen dioxide and that nitrogen dioxide then reacted to water to get nitric acid.
2 Cu(NO3)2 → 2 CuO + 4 NO2 + O2
2 NO2 + H2O → HNO2 + HNO3
The above process is called the Ostwald process.
Nitric acid can behave as a strong oxidizing agent. To incorporate the nitro group in any organic synthesis nitric acid is used widely.
1. How to draw the HNO3 lewis structure?
The HNO3 lewis structure is quite different from the other covalent molecule because it has two central atoms one is N and the other is an O atom. N and O both are p block elements, so they have to follow the octet rule in the drawing of the HNO3 lewis structure. Let us consider the HNO3 lewis structure.

Step 1 – In the first step of the HNO3 lewis structure, we should count the valence electrons for every atom present in it. The electronic configuration of H is 1s1. So it has only one electron and this electron is used for its valence electron and via this single electron, H can form a bond. We know O is the group 6thelement and p block element so its last orbital should be p orbital and the electronic configuration of O is [He]2s22p4. So it has six electrons in its valence shell which can be used for bond formation.
Now for N, the electronic configuration is [He]2s22p3, so it has five electrons in its valence shell and the maximum number of bonds N can form is four. Now we add all the valence electrons in the HNO3 lewis structure and the total valence electrons for the HNO3 lewis structure is, 1+5+(6*3) = 24 electrons. There are three O atoms present and each O contains six valence electrons.
Step 2 – Now it is a most confusing step, that here we select the central atom, here N and O both are present at the central position and the electronegativity difference between both is very less. In the HNO3 lewis structure, all the atoms are not connected through N or O atoms, but the central atom should connect all the atoms.
That’s why here N and one O are considered central atoms and for this reason, we have to calculate the hybridization for two atoms separately and the values of hybridization of two atoms are different. Two atoms are experiences different environments.
Step 3 – In the HNO3 lewis structure, all the atoms are from the s and p block, so they should follow the octet rule. For the s block element, they try to complete only their s orbital because s is their valence orbital. the total number of electrons accumulated by the s orbital is 2. For p orbital, the total electrons accumulated is 6.
Now, according to the octet rule, the electrons needed for the HNO3 lewis structure are 2+(4*8) = 34 electrons. But the valence electrons in the HNO3 lewis structure is, 24, so the shortage of electrons is 34-24 = 10 electrons. These 10 electrons should be accumulated in the number of suitable bonds. So the bonds needed 10/2 = 5 bonds. So there is a minimum of five bonds are needed. To assign the five bonds we need to add a double bond between N and O.
Step 4 – In this step, we should connect every atom in the HNO3 lewis structure via the required number of bonds. There is one bond between terminal H and O, one bond between O of -OH group and N. then other three bonds are used between N and two O atoms, and there is a double bond between one O and N atoms.
Step 5 – In this last step, we add lone pairs and multiple bonds to complete the valency of atoms. O has six electrons in its valence shell and it forms two bonds, one with H and one with N, so it has four unpaired electrons which exist as two pairs of lone pairs. Other O atoms which form a double bond with N, it has the same four electrons in their valence shell and they exist as two pairs of lone pairs. The last O which makes a single bond with N forms a dative bond and six electrons exist after one bond formation so it gets a negative charge and two pairs of lone pairs.
2. HNO3 lewis structure shape
The shape of the molecule is dependent on the electron count and based on the central atom, but in the HNO3 lewis structure, there are two central atoms one is N and the other is O. for N the shape of the molecule is planar but concerning O the shape of the molecule is tetrahedral. The electron density is different for two atoms and the shape of the HNO3 lewis structure.

The HNO3 lewis structure in the gaseous form is planar. The same structure is also for a solid-state. The N-O bond distance in the nitro group is equal. The third N-O bond distance is longer and corresponds to a single bond. We know Nitro is a variable structure and there is a double bond character shown between two O and N atoms. The nitro group is tilted away from the H atoms by 20.
If we consider the VSEPR(Valence Shell Electrons Pair Theory), then we should count the electrons and consider the structure. But here the structure is different for different atoms. For the -OH oxygen their electron count is 8 including two lone pairs, so according to the VSEPR, it adopts a tetrahedral shape. But for the nitro group electron count is 6 so it adopts trigonal planar structure, where three o atoms are present in three vertices.
3. HNO3 valence electrons
For calculating the total valence electrons for the HNO3 lewis structure, we should count individual valence electrons for each atom. There are three O atoms, one N and one h atom are present. The environment of three N atoms is different and therefore their valence electrons are also different.

From the electronic configuration of N it is evident that there are five electrons are present at the valence shell in N. O is group 6thelement so it has six electrons in its valence shell which are ready for bond formation. But O forms only one bond with N and the rest of the two electrons exist as lone pairs. H has only one electron and this one electron is the valence electron for the H atom.
So the total number of valence electrons of the HNO3 lewis structure is 1+5+(6*3) = 24 electrons.
4. HNO3 lewis structure lone pairs
In the HNO3 lewis structure, only lone pairs are available on the O atoms, N forms a dative bond with O in the nitro group then it has lone pairs otherwise not.
For counting the lone pairs, we have to check the available electrons in the valence shell of every atom after bond formation. H has only one electron, so H can’t contain lone pair. Now O has six electrons in its valence shell and after two sigma bond formation, it has the remaining four electrons. These four electrons exist as two pairs of lone pairs over O in the -OH groups.
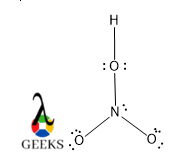
For the nitro groups, there are two O atoms present in the HNO3 lewis structure, One O is formed a double bond with N to complete the octet, so it has four electrons and again the same case arises. This O atom has two pairs of lone pairs. Now the other O atoms form a variable bond with N, there is not possible for two double bonds for N, because N cannot show pentavalent, so there is only one possible way that O makes a single bond with N or forms a dative bond.
A dative bond is a coordination covalent bond. The electron density is reside more towards the O site. That O also carries a negative charge and contains three pairs of lone pairs. Accounting for N is contain a positive charge.
So the total number of lone pairs over the HNO3 lewis structure is 2+2+3=7 pairs of lone pairs, otherwise, six pairs of lone pairs if they do not form a dative bond.
5. HNO3 lewis structure formal charge
From the HNO3 lewis structure, we can say that there is a charge present over N and O in the nitro group. By calculating the formal charge, the charge over every atom should be predicted. It is a hypothetical concept accounting for the same electronegativity of each atom in the HNO3 lewis structure.
The formula we can use to calculate the formal charge, F.C. = Nv – Nl.p. -1/2 Nb.p.
Where Nv is the number of electrons in the valence shell or outermost orbital, Nl.p is the number of electrons in the lone pair, and Nb.p is the total number of electrons that are involved in the bond formation only.
The environment of three O atoms should be different so, we have to calculate the formal charge individually for all the atoms.
The formal charge over the H atom is, 1-0-(2/2) = 0
The formal charge over the O atom in -OH group = 6-4-(4/2) = 0
The formal charge over the N atom is, 5-1-(8/2) = 0
The formal charge over the O in the nitro group is, 6-4-(4/2) = 0
From the formal charge calculation, we cannot say that there is a dative bond between N and O in the nitro group. Overall the molecule is neutral.
6. HNO3 lewis structure angle
The bond angle of the HNO3 lewis structure is different due to two different central atoms and two shapes of the molecule being present. The hybridization of two atoms is different and the environment is also different. So, in the HNO3 lewis structure, there is two bond angles are observed concerning N and O atoms.
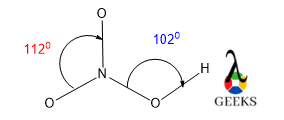
For the Nitro group in the HNO3 lewis structure, there is only three atoms are present surrounding the central N atom, and there are no lone pairs over the N atom. According to the VSEPR theory, if the molecule AX3 is without lone pair over the central atom, then the bond angle should be 1200. But here the lone pairs of three O atoms are present.
So if the bond angle goes 1200, then there is massive lone pairs-lone pair repulsion occurs. So to minimize this kind of repulsion, N changed its bond angle to 1120. This value is also for the presence of three electronegative atoms and the s character of the hybridization value increases.
Now for the other O atom, there is one H and one N atom are present surrounding that O atom. There are also two pairs of lone pairs present. So this shape is similar to a water molecule, like a V shape, so the bond angle is nearly 1040, because of lone pairs repulsion and the presence of one electronegative atom N.
7. HNO3 lewis structure octet rule
In the HNO3 lewis structure, there are s and p block elements present, so they have to follow the octet rule. The octet rule for s block elements is completing the s orbital via two electrons and for p block elements they complete their octet via eight electrons. Because p orbital can accumulate a maximum number of six electrons.

In the HNO3 lewis structure, we should check each atom to complete its octet. The H atom has only one electron in its s orbital and it needs one more electron to complete its octet. Via the bond formation with the O atom, H and O share one electron each, and therefore H can complete its octet via the one electron from the O site.
Now for the O of -OH group, it has six electrons in its valence shell, as it is the group 6th element. So it required two more electrons in its valence shell to complete its octet. Now this O makes a bond with H and N atoms sharing two electrons. So it completes its octet by gaining two electrons from N and O, which are shared in the bond formation.
Now N has five electrons in its valence shell and it needs three more electrons in its valence shell to complete its octet. So it makes a bond with three O atoms and shares three electrons with them. Now N can complete its octet too via gaining those three electrons from three O atoms which are shared in the bond formation.
Two O atoms of the nitro group make a double bond at a time with the N atom and hence they can complete their octet too, via sharing two-electron in the double bond.
8. HNO3 lewis structure resonance
In the HNO3 lewis structure resonance will be observed because there more electron clouds are present within the molecule, which can be delocalized over the different skeleton forms of the HNO3 lewis structure. The molecule shows a positive and negative charge within it and there is more number of lone pairs present, so they can be delocalized, because the octet of the N and ketonic O is not complete somehow.

all the above three structures are different skeleton forms of HNO3 lewis structure or we can say that they area resonating structure. Among the three, structure I and structure II are similar, the negative charge dispersed over the two O atoms in the nitro group. These two structure is most contributing because all three structure contains the same number of covalent bond, but in these two structure the most electronegative atom O gets a negative charge and the less electronegative atom N gets a positive charge.
In structure III, the number of covalent bonds is the same but here N gets a positive charge is ok but O also gets a positive charge which is a destabilization factor. O is a more electronegative atom and the positive charge over it is a destabilization factor.
9. HNO3 hybridization
The hybridization of the HNO3 lewis structure is different because it has two central atoms and we should predict the hybridization of two central atoms individually. The energy of 1s orbital of H and 2p orbital of O is not equivalent so they undergo hybridization to form equivalent orbital. Similarly, the N atom also undergoes hybridization from a hybrid orbital with three O atoms.
We calculate the hybridization of the HNO3 lewis structure by using the following formula,
H = 0.5(V+M-C+A), where H= hybridization value, V is the number of valence electrons in the central atom, M = monovalent atoms surrounded, C=no. of cation, A=no. of the anion.
When we find the hybridization of N in the HNO3 lewis structure, there are three electrons for N and three O atoms are present at the surrounding position.
So, the hybridization of central N in the HNO3 lewis structure is ½(3+3+0+0) = 3 (sp2)
For the O atoms, the valence electrons are 6 and one N and one H atom are present.
So the hybridization of O in the HNO3 lewis structure is, ½(6+2+0+0) =4 (sp3)
| Structure | Hybridization value | State of hybridization of central atom | Bond angle |
| Linear | 2 | sp /sd / pd | 1800 |
| Planner trigonal | 3 | sp2 | 1200 |
| Tetrahedral | 4 | sd3/ sp3 | 109.50 |
| Trigonal bipyramidal | 5 | sp3d/dsp3 | 900 (axial), 1200(equatorial) |
| Octahedral | 6 | sp3d2/ d2sp3 | 900 |
| Pentagonal bipyramidal | 7 | sp3d3/d3sp3 | 900,720 |
From the above table of hybridization, we can conclude that if the hybridization value is 4 then the central atoms is sp3 hybridized and if the number of orbitals are involved in hybridization is three then the atoms is sp2 hybridized.
Let’s understand the hybridization of N and O atoms separately.
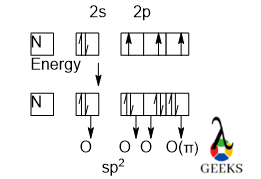
It is evident from the hybridization diagram that we cannot consider the π bond in the hybridization. We consider only the sigma bond with O atoms, N forms a three-sigma bond with three O atoms undergoing sp2 hybridization. S orbital cannot participate in multiple bond formation, so we consider p orbital electron for forming a double bond.

From the hybridization diagram, we can tell that the lone pairs over the O atoms are also involved in the hybridization. For this reason, the shape around that O atom is tetrahedral and this also interns the value of the bond angle is near 109.50.
10. HNO3 solubility
In the HNO3 lewis structure, we can say that there is charge separation observed and this makes the molecule slightly polar and ionic, so it can soluble in a different polar solvent. HNO3 is a very good solvent for different metals. It forms aqua regia, which is a good solvent for the soluble gold metal. HNO3 is soluble in the following solvents,
- Benzene
- Ethanol
- Water
- CCl4
11. Is HNO3 soluble in water?
In the HNO3 lewis structure, we see some polarity in this molecule, so it is soluble in a polar solvent. HNO3 is not soluble in water directly, it is miscible in water, the density of water and HNO3 is different but it has a polar character and is ionized in water and gets miscible in an aqueous solution.
12. Is HNO3 polar or nonpolar?
The presence of different atoms and the shape of the HNO3 lewis structure make the molecule polar. Although the electronegativity of N and O is almost close the electronegativity of ketonic Oxygen and alcoholic Oh is different. The shape of the molecule concerning N is planar but concerning O it is tetrahedral and due to its unsymmetric shape it makes the molecule polar. Due to the unsymmetric structure, the dipole moment of the molecule is not canceled out and the molecule has some resultant dipole moment.

The direction of the dipole moment is from N to O, as N gets a positive charge and the electropositivity is increased here and O gets a negative charge, and the electronegativity increases, and for this reason, the difference between electronegativity is higher and dipole moment works. from the structure, we can say the dipole moment is not canceled out or not exactly opposite to each other. So it has a valid dipole-moment value and makes the HNO3 lewis structure polar.
13. Is HNO3 an electrolyte?
Yes HNO3 is an electrolyte it dissolves in water and makes the aqueous solution ionic.
14. Is HNO3 a strong electrolyte?
HNO3 is a strong electrolyte, cause when it gets solvated in an aqueous solution is it ionized in H+ and nitrates ions. The mobility of the H+ ion is very high and for this reason, the HNO3 lewis structure is a strong electrolyte.
15. is HNO3 acidic or basic?
HNO3 is strongly acidic, when it is dissolved in water it released H+ ions easily and makes a stronger acidic.
16. Is HNO3 a strong acid?
HNO3 is a strong acid, it can release H+ ion easily because there is an electronegative O atom is present and for the electronegativity, it pulls electron density toward itself and making O-H bonds weaker and H+ easily cleaved, if a molecule release H+ easily then the acidic nature of this molecule is very high and making a stronger acid.
17. Is HNO3 an arrhenius acid?
If a molecule gives H+ ion then it is called Arrhenius acid and HNO3 easily releases an H+ ion, so HNO3 is an Arrhenius acid.
18. Is HNO3 a lewis acid?
HNO3 can acts as lewis acid because it can accept electron density in N atom because it is positively charged.
19. Is HNO3 stronger than HNO2?
HNO3 is a stronger acid than HNO2 because the conjugate base of HNO3 is NO3- which is more stabilized due to conjugation as compared to the conjugate base of HNO2, which is NO2-. So more stable the conjugate base stronger the acid is.
20. Is HNO3 stronger than H2SO4?
HNO3 is less strong than H2SO4, as H2SO4 is dibasic acid and also the conjugate base of H2SO4 is SO42-, which is a more stable conjugate base due to better overlap between O and S as compared to the conjugate base of HNO3, which is NO3-. SO HNO3 is a weaker acid than H2SO4.
21. Is HNO3 a conjugate acid?
No HNO3 is itself an acid and it is not a conjugate acid of another molecule.
22. Is HNO3 a conjugate base?
HNO3 is an acid, so it has a conjugate base and the conjugate base of HNO3 is NO3-, which is more stable and makes the HNO3 stronger acid.
23. Is HNO3 diprotic?
There is one hydrogen atom present and it can be donated, so HNO3 is monoprotic, not diprotic.
24. Is HNO3 binary or ternary?
HNO3 is a ternary oxoacid.
25. Is HNO3 a buffer?
When HNO3 reacts with a weak base then it can form a buffer of strong acid, otherwise, it is an acid.
26. Is HNO3 a salt?
HNO3 is an acid and when it reacts with a strong base then it forms salt and water molecule.
27. Is HNO3 conductive?
HNO3 is a conductive agent when it is soluble in water it can carry electricity.
28. Is HNO3 corrosive?
HNO3 is highly corrosive.
29. Is HNO3 hydrogen bonding?
There is no H bonding observed in the HNO3 structure.
30. Is HNO3 linear?
HNO3 is not linear it is planar and tetrahedral in shape.
31. Is HNO3 paramagnetic or diamagnetic?
HNO3 has only one unpaired electron over the N atom and it is paramagnetic.
32. Is HNO3 amphoteric?
HNO3 is a strong acid and donates H+ ions only so it is not amphoteric.
33. Is HNO3 a dehydrating agent?
HNO3 is a dehydrating agent, it can remove water molecules.
34. Is HNO3 gas?
The physical state of HNO3 is liquid.
35. Is HNO3 electrophile?
The nitronium ion in HNO3 acts as an electrophile.
36. Is HNO3 hygroscopic?
The dilute HNO3 is hygroscopic but the concentrated form is not.
37. Does HNO3 have a charge?
HNO3 is not a charged molecule but there is a negative charge dispersed between ketonic groups in HNO3.
38. Is HNO3 heavier than air?
The density of HNO3 is much higher than in air.
39. Is HNO3 liquid or aqueous?
The physical state of HNO3 is liquid.
40. Is HNO3 monobasic?
HNO3 has one replacable H+ ion is present so it is monobasic acid.
41. Is HNO3 acid metal?
No HNO3 is not a metallic compound.
42. Is HNO3 an oxidizing agent?
In HNO3 the oxidation number of N is high and it can be decreased so it behaves as a stronger oxidizing agent.
43. Is HNO3 radical
No, HNO3 is not radical, it can be ionized.
44. Is HNO3 a reagent?
Yes in organic synthesis it uses as a nitro group incorporated reagent.
45. Is HNO3 triatomic?
Yes HNO3 is triatomic as it contains N, O, and H atoms.
46. Is HNO3 volatile?
Yes, it is more volatile than H2SO4.
47. why HNO3 is yellow?
When HNO3 lasted for a long time, then oxide of N is deposited and for this reason, the color of HNO3 is yellow.
Conclusion
HNO3 is a monobasic strong acid in liquid form. It is used as a reagent in different chemical syntheses.
Also Read: