Silicon tetrafluoride (SiF4) has a central silicon (Si) atom with 4 valence electrons, forming single bonds with four fluorine (F) atoms, each contributing 7 valence electrons. The Lewis structure shows four Si-F bonds and no lone pairs on silicon, using 8 bonding electrons. SiF4 adopts a tetrahedral geometry with bond angles of approximately 109.5°, characteristic of sp³ hybridization. The molecule is nonpolar due to its symmetrical shape, despite the high electronegativity of fluorine (3.98). This structure and the strength of the Si-F bonds significantly influence SiF4’s chemical properties, including its reactivity and role in the semiconductor industry.

Let us discuss the following point in this article
- how to draw lewis structure for SIF4
- SIF4 lewis structure lone pairs
- SIF4 lewis structure shape
- SIF4 lewis structure octet rule
- SIF4 hybridization
- SIF4 lewis structure resonance
- SIF4 polar or nonpolar
- SIF4 lewis structure formal charges
- SIF4 lewis structure shape
how to draw lewis structure for SIF4
The shape of a molecule depends upon the repulsion between the valence electron bond pair or nonbonding pair. In the SIF4 molecule, the four fluorine atoms are surrounded by a central silicon atom. The Silicon atom has four unpaired electrons,
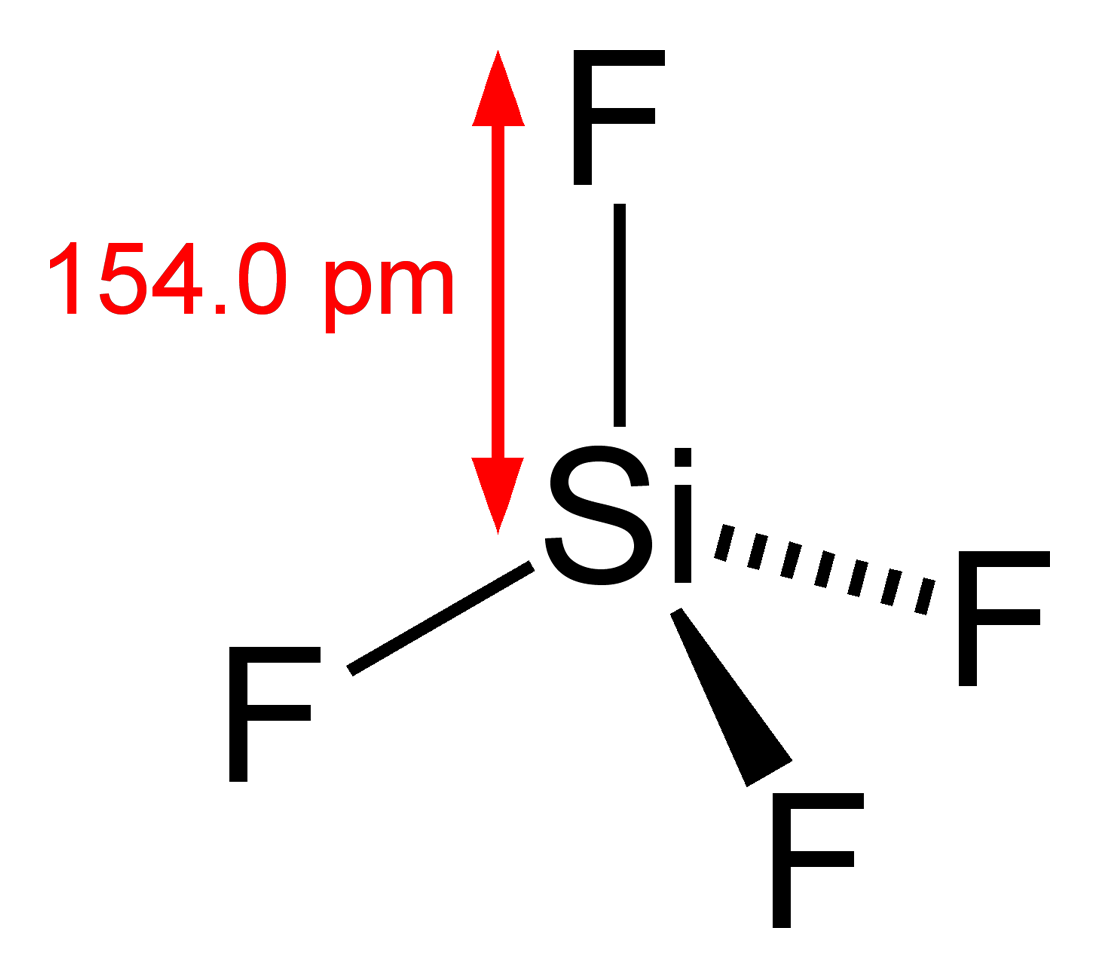
According to the octet rule silicon is paired with four other atoms to complete its octet. Hence form four Si-F bonds. According to VSEPR theory, the SiF4 molecule shows tetrahedral geometry, with a bond angle of 109.5o and bond length of 154 pm.
SIF4 lewis structure lone pairs
Valence electron of one silicon atom= 4×1=4
Valence electron of four fluorine atoms = 7×4=28
Total valence electrons = 32 there are 16 pairs of electrons required for a stable Lewis structure.
In the above structure total, we have 16 electron pairs with four Si-F bonds So 12 electrons remain which are denoted by lone pairs, for each fluorine atom there are three lone pairs hence total of 12 lone pairs of electrons are present around the silicon atom,
and silicon has zero pair of the electron because all 12 electron pairs are present around the four fluorine atom.
SIF4 lewis structure shape

SIF4 lewis structure octet rule
In the above Lewis structure, silicon and fluorine do not have any charges and the central silicon atom completes its octet therefore this structure is a stable Lewis structure. For a stable Lewis structure, all the atoms in the molecules present must satisfy the octet rule,
octet rule states that to attain a stable configuration valence shell of an atom contains eight electrons which resemble an electronic configuration of the nearest noble gas.
In the SiF4 molecule, the fluorine atom requires only one electron to complete its octet while the silicon atom requires four electrons to complete its octet and become stable. silicon and fluorine atom shares one electron with each other and completes their octet, hydrogen having two valence electron and silicon having eight valence electrons in this way they complete their octet.
SIF4 hybridization
Hybridization is a process in which the atomic orbitals of both the atoms in a molecule come together and combine with each other to form a hybrid orbital by direct overlapping sigma bond is formed while side to side parallels overlap forming a pi bond.
In SiH4 molecule the electronic configuration of silicon is,
Si: 1s2 2s2 2p6 3s2 3p2
Si: [Ar] 3s2 3p2

From the above diagram, the s orbital and three p orbital come together and combine to form 4 hybridized 3p3 orbitals these 4 hybrid orbitals form four sigma bonds with four hydrogen atoms. Therefore, the hybridization for Si is sp3 in SiH4.
SIF4 lewis structure resonance
Resonance is a chemical phenomenon in which whole properties of molecule are not not able to explain with a single structure. There are many canonical structures involved. But not every molecule can exhibit resonance.
SiF4 lewis structure does not exhibit resonance because there is no delocalization of electrons and there is the presence of single bonds. So there is no movement. Even though there is the presence of lone pairs of electrons, delocalization disturbs the stability factor. Hence there are no resonating structures of the SiFl4 lewis structure
SIF4 polar or nonpolar
The four fluorine atoms are surrounded by a central silicon atom in the SiF4 molecule, the electronegativity of the silicon atom is 1.90 and that of electronegativity of the fluorine atom is 3.98 the difference between the electronegativity of fluorine and silicon is 2.08 ,
this much electronegativity difference between silicon and fluorine indicates that the electron pairs are strongly attracted towards fluorine atom hence Si-F bond in SiF4 molecule is polar. the more electronegativity difference between them indicates that the electron pair is strongly attracted to the fluorine atom hence Si-F bond is highly polar.
SIF4 lewis structure formal charges
In silicon tetrafluoride molecule number of actual charges corresponds to total formal charges. The formal charges are calculated by the SiF4 Lewis dot structure. Which are calculated by the following formula,
The formal charge on Si atom of SiF4 molecule = valence electron of Si- lone pair of silicon -1/2 (bond pair of electrons)
According to the formal charge calculation formula, the silicon atom has 4 valence electrons, eight bond electrons, and no lone pair. hence , In SiF4 molecule Therefore, formal charge on silicon atom of SiF4 molecule = (4- 0-(8/2)) =0
Hence the formal charges in the SiF4 molecule are zero.
SIF4 lewis structure shape
Tetrahedral structure of SiF4 Shown below:
Frequently Asked Questions
What is the Lewis structure of SiF4?
Answer : In the Lewis structure of SiF4 molecule central silicon atom is surrounded by four fluorine atoms, it forms four Si-F bonds, all fluorine atoms have three lone pairs on each.
What Are the Similarities and Differences Between the Lewis Structures of XeO3 and SIF4?
The xeo3 lewis structure explained reveals that both XeO3 and SiF4 have central atoms surrounded by electron pairs and bond pairs. However, the key difference lies in the number of electron pairs and bond angles. XeO3 has 3 bond pairs and 2 lone pairs, resulting in a trigonal pyramidal shape with bond angles of approximately 109.5°. On the other hand, SiF4 has 4 bond pairs and no lone pairs, giving it a tetrahedral shape with bond angles of 109.5°.
How do you predict the shape of SiF4?
Answer: Shape of SiF4 is predicted by VSEPR theory, Central silicon atom has four valence electrons hence it attached with four fluorine atoms and form tetrahedral geometry.
What is the significance of Lewis structure?
Answer: It defines the nature of bond and position of atoms of the molecule which are connected in the molecule. The representation of molecules in Lewis electron dot structure or just a Lewis structure is in honor of the American chemist Gilbert Newton Lewis.
Also Read:
- Ncl2 lewis structure
- Co2 lewis structure
- Bao lewis structure
- Sncl2 lewis structure
- H2so4 lewis structure
- Xecl2 lewis structure
- Chf3 lewis structure
- Na2so4 lewis structure
- Bf3 lewis structure
- H2o2 lewis structure

Hi….I am Darshana Fendarkar, I have completed my Ph.D. from the University of Nagpur. My area of specialization is Inorganic Chemistry.
I have an experience as a Chemist at Earthcare Pvt. Ltd. Also I have 2 years of experience in teaching. Currently, I am working with Lambdageek as a Subject Matter Expert.


Hi Fellow Reader,
We're a small team at Techiescience, working hard among the big players. If you like what you see, please share our content on social media. Your support makes a big difference. Thank you!