This article is regarding about HSO3- lewis structure and other important characteristics and features. Let’s start with the HSO3- lewis structure.
HSO3- is a non-metal sulfite molecule. It is an oxoanion of sulfur. It is also a conjugate base of sulfurous acid. the central S is sp3 hybridized like in sulfurous acid. One of the -OH bonds is replaced in sulfurous acid by O–, as H+ is released from sulfurous acid. It can act as both acids as well as base as it releases H+ as well OH– under suitable conditions.
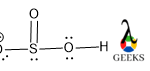
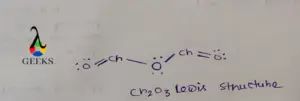
Central S is connected via one ketonic O, one -OH group, and other O bearing a negative charge. The negative charge can be delocalized between S and O atoms as they can accumulate the negative charge being an electronegative atom.
Some facts about HSO3-
There is always tautomerism existing between bisulfite anions. This phenomenon is observed in the NMR spectroscopy. One tautomer has double bonded O and the other has -OH group. HSO3- can be prepared from sulfurous acid by the proton loss or from calcium bisulfite by loss of calcium cation.
H2SO3 = H+ + HSO3–
CaHSO3 = Ca+ + HSO3–
Again, when sulfur dioxide is reacted with a basic solution of a strong base then it gives HSO3–.
SO2 + OH– = HSO3–
HSO3– is the conjugate base of sulfurous acid having pka 6.97, so it is less basic as well as less acidic. As HSO3– is a very weak acid so its conjugate base is SO32-.
HSO3– = H+ + SO3–
Bisulfite is also a good reducing agent, it can give hydrogen easily.
2HSO−3 + O2 → 2SO2−4 + 2H+
1. How to draw HSO3- lewis structure?
HSO3- lewis structure plays a crucial role in the prediction of the different covalent characteristics of the anion. So, we try to learn how HSO3- lewis structure can be drawn.
First, we count the total valence electrons for the HSO3- lewis structure. The three constituents of HSO3- lewis structure are S, O, and H. the valence electrons for S, O, and H are 6,6, and 1 respectively. So, the total valence electrons for HSO3- lewis structure is (4*6) + 1 + 1 =26 electrons.
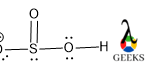
The presence of an extra negative charge is a sign of the presence of an extra electron and so we add 1 to the valence electrons.
Now we choose S as the central atom, as it has larger and less electronegative than O.
According to the octet rule the electrons required for HSO3- lewis structure is, 4*8 + 2 +1 = 35 electrons, but the valence electrons of HSO3- lewis structure are 26 electrons. So, shortage of electrons are 35-26 = 9 electrons.
Those shortage 9 electrons will be accumulated by a suitable number of bonds that is 4 bonds and 1 extra electron resides as a negative charge.
As O is more electronegative so negative charge on O is the most favorable case. After assigning all the bonds we should assure all the atoms should be satisfied by their valency.
O is bivalent atoms, so satisfying its valency we add a double bond between S and O. All the lone pairs are assigned over S and o atoms as they contain more electrons in their valence shell.
2. HSO3- lewis structure shape
HSO3- lewis structure shape is as like H2SO3 molecular shape that is trigonal pyramidal. But the molecular geometry of HSO3- lewis structure is tetrahedral according to VSEPR theory and hybridization value.
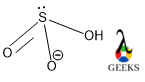
In the HSO3- lewis structure central S undergoes sp3 hybridization along with its lone pair and makes π bond with the 3d orbital. So, according to VSEPR (Valence Shell Electrons Pair) theory, the molecule should adopt tetrahedral geometry to avoid any kind of steric repulsion as it is a tetracoordinate molecule, but the shape of the molecule is trigonal planar.
In the shape we check the geometry without the lone pair, only bond pairs are involved and there are three bond pairs responsible for the geometry and the best geometry is trigonal pyramidal.
3. HSO3- valence electrons
The total valence electrons for HSO3- lewis structure are 26. These 26 electrons are the summation of individual atoms present in the anion.
The central atom S has six valence electrons because it belongs to group 16th, among them two are from 3s and four are from 3p orbital.
O has also six valence electrons as it belongs to the group VIA of the periodic table two electrons of O are from the 2s orbital and the rest four electrons belong to another valence shell 2p orbital.
H has only one valence electron as it is group IA and 1st-period element. The negative charge over the anion is also counted as one electron.
So, the total valence electrons present in the HSO3- lewis structure are (6*4) + 1 + 1 = 26.
4. HSO3- lewis structure lone pairs
Only S and O contain lone pairs in the HSO3- lewis structure. The total lone pairs are the summation of the individual lone pairs present over O and S atoms.
S has six valence electrons but S has four bond pairs in the HSO3- lewis structure by sharing four electrons. So, the remaining two valence electrons exist as one lone pair over the S.
O has also six valence electrons and two O have two bond pairs via sharing two electrons and the rest of the four valence electrons as two pairs of lone pairs.
But one O has only one bond pair with S and it also contains one extra electron in its valence shell. So, it gets a negative charge and now it has seven electrons and only one bond pair via sharing one electron.
So, the remaining six electrons exist as three pairs of lone pairs for that O atom.
So, the total lone pairs for the HSO3- lewis structure are 1+2+2+3 =8 pairs of lone pairs.
5. HSO3- lewis structure octet rule
Every atom after bond formation will follow the octet rule for stabilization and gain the noble gas configuration. So, every individual atom in the HSO3- lewis structure also obeys the octet rule for stabilization.
The electronic configuration of S is [Ne]3s23p4. So, from the electronic structure of S, it is shown that it has six electrons in its outermost orbital which are 3s and 3p. It is a group VIA 3rd-period atom of the periodic table, so it has six valence electrons.
S needs two more electrons in its 3p orbital so its 3p orbital is filled because the p orbital can contains a maximum of six electrons as it has three subsets. After gaining two electrons in the p orbital of S, its p orbital is filled like the nearest noble gas and is also stable.
Then S has six electrons in the p orbital and two electrons in the s orbital, so S would have eight electrons in its valence orbital and complete its octet.
In the HSO3- lewis structure S makes three sigma and one π bonds with H and O atoms respectively. One electron is to be promoted to a vacant 3d orbital and that electron is formed a π bond. Now S has three unpaired electrons in its 3p orbital and these three electrons make bonds via sharing electrons.
Now S has six paired electrons in its 3p orbital and two electrons in the 3s orbital. So, finally, S has eight electrons in its valence shell that is in 3s and 3p orbitals and completes its octet like a noble gas.
O has electronic configuration [He]2s22p4, so it has also six electrons in its valence orbital which are 2s and 2p. As O belongs to group 16th 2nd-period of periodic table so it also has six valence electrons like S. O has more than half-filled in its 2p orbital and needs two more electrons for the complete octet rule.
Two O atoms formed two bonds in the HSO3- lewis structure by using two electrons and now O has three paired electrons in its p orbital and it has two electrons in its 2s orbital. So, O has eight electrons now and complete its octet also.
One O atom contains a negative charge over it and has five electrons in its 2p orbital and needs one more electron.
That O formed a single bond with S by sharing one of its electrons and now it also has six electrons in its 2p orbital and two electrons in the 2s orbital already. So, that O also has eight electrons in its valence orbital like group 18th element and completes its octet to gain the stabilization
H has only one electron in 1s orbital, and s orbital contains a maximum of two electrons, so it needs one more electron so it can form an electronic configuration like He. H forms a single bond with O shares one electron and its 1s orbital is completed.
6. HSO3- lewis structure formal charge
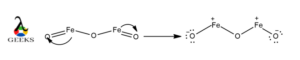
As HSO3- lewis structure contains a negative charge over it so we have to calculate the formal charge to show which atom contains a negative charge. We assume the same electronegativity for all atoms present in the molecule.
The formula we can use to calculate the formal charge, F.C. = Nv – Nl.p. -1/2 Nb.p.
Where Nv is the number of electrons in the valence shell or outermost orbital, Nl.p is the number of electrons in the lone pair, and Nb.p is the total number of electrons that are involved in the bond formation only.
The formal charge over S is, 6-2-(8/2) = 0
The formal charge over O is, 6-4-(4/2) =0
The formal charge over O is, 6-6-(2/2) = -1
The formal charge over H is, 1-0-(2/2) = 0
SO, one of the O atoms contains a negative charge over it because O has a formal charge of value -1.
7. HSO3- lewis structure angle
The O-S-O bond angle in HSO3- lewis structure is larger than expected. It should be around 109.50 as central S is sp3 hybridized and geometry-like tetrahedral.

The bond angle is depending on the hybridization as well as VSEPR theory. So, naturally, the AX3 type molecule having lone pair shows tetrahedral geometry and the bond angle will be 109.50. lone pairs required more space and for that reason, geometry will be tetrahedral.
But if there is a deviation factor present within the molecule then the bond angle will be changed and will show the exception of VSEPR theory. In the HSO3- lewis structure there is a lone pair along with a double bond. So, there is massive lone pair-bond pair repulsion occurs. To minimize that repulsion molecule changes its geometry to trigonal pyramidal.
But the bond angle for trigonal planar is 1200. But there is a lone pair and bond pair repulsion the central molecule aligns the bond angle lower than 1200 which is 1130 for stable configuration, but the bond angle is higher than 109.50.
8. HSO3- lewis structure resonance
Due to the presence of excess electron density in the HSO3- lewis structure there will electrons cloud delocalization will be occurred. This phenomenon refers to resonance.
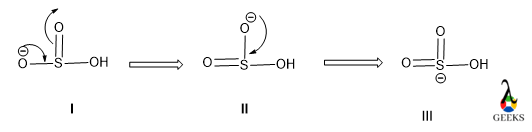
There will be three different resonating structures of HSO3- lewis structure that will be possible. Among all only structure III is the most stable canonical form of the molecule as it contains a higher number of covalent bonds so it is the most stable and most contributing as well.
The structure I and II are similar so, they have lesser stability than structure I.
9. HSO3- hybridization
In the HSO3- lewis structure central S atom should be sp3 hybridized. There are different atoms present with different orbitals having different energy. So, they undergo hybridization to form hybrid orbitals having equivalent energy to form a stable bonding.
The hybridization of N is calculated by the following formula,
H = 0.5(V+M-C+A), where H= hybridization value, V is the number of valence electrons in the central atom, M = monovalent atoms surrounded, C=no. of cation, A=no. of the anion.
So, the hybridization of central S is, ½(6+1+1) = 4(sp3)
| Structure | Hybridization value | State of hybridization of central atom | Bond angle |
| Linear | 2 | sp /sd / pd | 1800 |
| Planner trigonal | 3 | sp2 | 1200 |
| Tetrahedral | 4 | sd3/ sp3 | 109.50 |
| Trigonal bipyramidal | 5 | sp3d/dsp3 | 900 (axial), 1200(equatorial) |
| Octahedral | 6 | sp3d2/ d2sp3 | 900 |
| Pentagonal bipyramidal | 7 | sp3d3/d3sp3 | 900,720 |
So, we can conclude from the above table if the hybridization is involved within 4 orbitals then the central tom should be sp3 hybridized.
Now we can understand the hybridization of S and the bond formation.

Again, from the box diagram, we can see that one of the electrons of S from the p orbital is get promoted to the vacant 3d orbital and that electron is forming a π bond with O which is not involved in hybridization. So, in the HSO3- lewis structure there will be one dπ-pπ bonding will be formed.
10. HSO3- solubility
HSO3- is mostly water-soluble but it is also soluble in the following solutions,
- CCl4
- Methanol
- Benzene
- Toluene
11. Is HSO3- soluble in water
Yes, HSO3- is water soluble.
The molecule is an anion and for this reason, it has some polarity and for this reason, it is soluble in polar solvents like water (like dissolves like).
12. Is HSo3- an acid or base?
HSO3- acts as both acids as well as the base.
HSO3- is a conjugate base of H2SO3, so here it can act as a base and can donate -OH.
But in an aqueous solution HSO3- can release H+ and acts as an acid. its conjugate base is SO32-.
13. Is HSO3- a strong acid?
No, HSO3- is a very weak acid.
The pka value of this molecule is very high and positive making it weak and acidic. In water solution, it dissociates very slowly. But its conjugate acid sulfurous acid is a moderately strong acid.
14. Is HSO3- a strong base?
No, HSO3- is not a strong base.
The pka value of HSO3- is nearly neutral. So, neither it is a strong acid nor neither is it a very strong base.
15. Is HSO3- a bronsted base?
No, HSO3- is not a Bronsted base.
It can be thought that HSO3- can be accepted as a proton or H+ easily but after accepting proton it is transferred to sulfurous acid. So, once it accepted the proton but after accepting proton it will be no longer a base change to an acid.
16. Is HSO3- aqueous?
No. HSO3- is not aqueous.
It is a colorless liquid in the physical state but in an aqueous solution is dissociated its proton is very slowly and no longer stays in its original form.
17. Is HSO3- a lewis acid?
Yes, HSO3- acts as a lewis acid.
S has an energetically accessible vacant 3d orbital. So, lone pairs from the suitable lewis base can be accepted there and make HSO3- as lewis acid.
18. Is HSO3- neutral?
No, HSO3- is charged anion.
There will be a negative charge present over the molecule, actually more precisely the negative charge is on the O atom. So, the molecule is an acid radical.
19. Is HSO3- polar or nonpolar?
HSO3- is a polar molecule.
There is a charge difference between S and O atoms. So, a net dipole moment will flow from the S to O site and due to the asymmetric shape of the molecule there is no chance of canceling out the dipole moment and the molecule has a resultant dipole moment. So, HSO3- is a polar molecule.
20. Is HSO3- a conjugate acid or base?
HSO3- is both con jugate acid as well as the conjugate base.
HSO3- is the conjugate base of sulfurous acid. whereas it is an acid itself, which conjugate base is SO32-. So, HSO3- can be both conjugate acid as well as a conjugate base.
21. Is HSO3- a polyatomic ion?
Yes, HSO3- is a polyatomic ion.
HSO3- consists of three types of ion, the negative charge is over the O atom. So, it is a polyatomic anion.
Conclusion
HSO3- is a conjugate base of sulfurous acid. but hSo3- itself is an acid but very weak. It slowly ionized in a water solution.
Also Read: