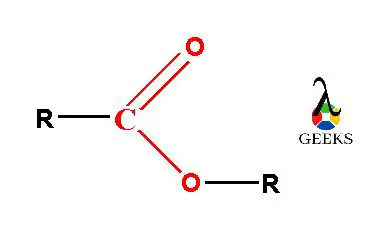
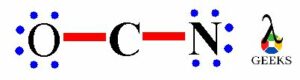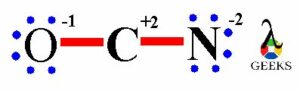Derivatives of carboxylic acids (-COOH) are known as esters. It has general chemical formula R-COOR’.
Easter formula R-COOR having two alkyl group in it one alkyl group attached with carbonyl (C=O) group and other alkyl group attached with oxygen atom adjacent to carbonyl group. Esters are considered as a polar solvent. Lower molecular weight esters get easily soluble in water. We are learning in this editorial about the topic of ‘Are esters soluble: 13 facts you should know’.
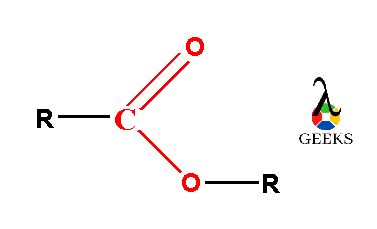
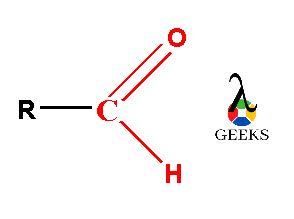
In the ester molecule, there are two alkyl groups either same or different linked with carboxylic (–COO) functional group. The carboxylic group (–COO) is consists of a carbonyl group (C=O) which is joined with one more oxygen atom forming –COO group. The two alkyl molecules are linked with this esteric –COO functional group.
But the long chain esters are not soluble in water only low carbon chain esters are soluble in water. In care of long chain ester molecules, the hydrogen bond gets broken by the long non-polar hydrocarbon chain and disrupts the bonding.
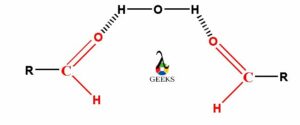
As the esters are not able to form hydrogen bonds with other ester molecules, so they can form hydrogen bond with water molecule. Even esters are able to make hydrogen bonds by its oxygen atom with the hydrogen atoms of water molecules.
What are esters soluble in?
Esters are soluble in most of the liquid solvents, especially esters are easily soluble in water and some organic solvents it depends on to the carbon atoms present in ester molecule. Easters are the compounds formed with two compounds i.e. carboxylic acids (R-COOH) and alkyl containing OH group (R-OH).
As the esters is containing carbonyl functional group containing double bond between carbon atom and oxygen atom. This carbonyl group is polar in nature. The carbonyl group containing carbon and oxygen atom which electronegativities says oxygen atom is more electronegative as compared to carbon atom. So, oxygen atom attracts the whole shared electron pair density in C=O double bond towards itself.
Thus, oxygen atom has a partial negative charge on it and partial positive charge produces on carbon atom of carbonyl group which is symbolized by the delta (δ) sign and creates a permanent dipole on it. So, due to this reason esters have permanent dipole-dipole forces within its molecules. The single covalent C-O bond in ester molecule also shows polar nature. So, the properties of ester molecule get influenced due to the presence of all these polar bonds. The complete polarity of ester molecule is shown in following image.
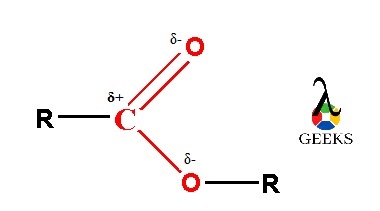
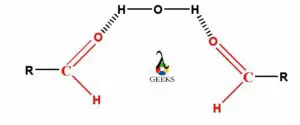
The esters can form hydrogen bond with water because the oxygen atom of carbonyl group present in ester molecule contains a lone electron pair. So, this oxygen atom containing lone electron pair can form hydrogen bonds with any of the two partial positive charge containing hydrogen atoms of water molecule. Therefore, as the water and esters both molecules are polar in nature, so the esters are readily soluble in water.
Due to this reason in our laboratory, the esters get filled later in the beaker which is already filled with water. So the esters formed an upper layer in a beaker before water layer without mixing in water. Because of all the reasons mentioned above and ester shows polar nature, the several esters are dissolved in water (H2O) and also dissolved in various organic solvents.
Which esters are soluble in water?
Ester molecules comprising of lowest chain of carbons in its structure are readily soluble in water. Specially the ester containing less than five carbon atoms in its molecule can easily form hydrogen bond with water and are easily soluble in water.
The names of the esters which are soluble in water are methyl formate (contains 2 carbon atoms), ethyl formate (containing three carbon atoms), methyl acetate (contains three carbon atoms) and ethyl acetate (contains four carbon atoms) are easily soluble in water.
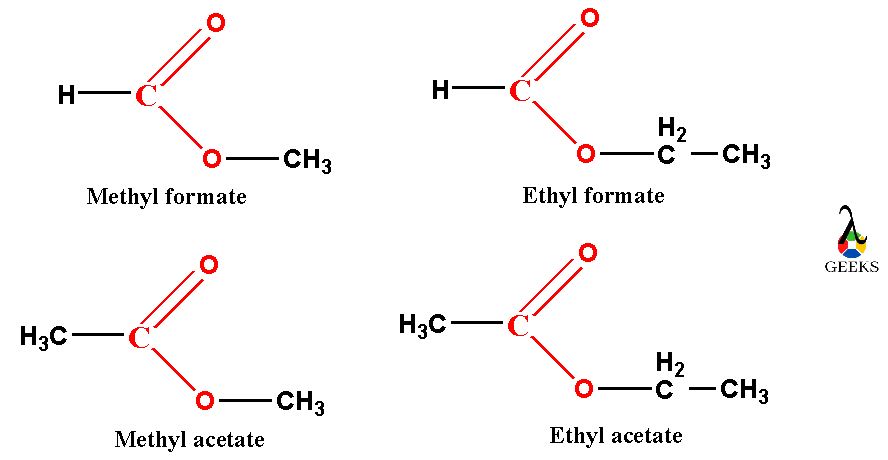
These all above esters can form hydrogen bond with water molecules and both are polar molecules in nature. So, all these above mentioned esters are only soluble in water.
Are esters soluble in organic solvents?
Yes, esters are soluble in various common organic solvents. As we know that solubility of esters in water or other polar solvents decreases as the carbon chain of esters increases.
As the long carbon chain present in esters, it becomes more and more non-polar in nature and unable to dissolve in polar solvents. But as the hydrocarbon chain increases in esters it is able to dissolve in various organic solvents.
The organic solvents like ammonia (NH3), sodium hydroxide (NaOH), hydrochloric acid (HCl), benzene (C6H6), amines, etc. Esters are soluble in such listed organic solvents. Even rather than this higher esters are used to prepare soaps by saponification process, also used to prepare fats and oils and esters are also used in preparation of various kinds of polythenes by reacting with various organic solvents.
Are esters soluble in polar solvents?
Yes, esters are soluble in polar solvents. Esters show both polar and non-polar nature. As the esters having small carbon chain means below five carbon atoms present in it are polar in nature. While the higher carbon chain containing esters i.e. contain more than five carbon atoms in its molecule are non-polar in nature.

As the lower carbon chain containing esters are polar in nature they can readily soluble in other polar solvents like water. Also they can make the weak hydrogen bonds with water molecules. The carbonyl group present in esters which is polar in nature and the oxygen atom of that carbonyl group containing lone electron pairs. So the oxygen atom of carbonyl functional group of esters can easily form hydrogen bonds with either of two hydrogen atoms in water molecule.
Are esters soluble in nonpolar solvents?
Yes, esters are soluble in various non-polar solvents. Non-polar solvents are the chemical compounds or molecules in which there is no dipole moment is present. The non-polar solvents do not contain any partial negative or positive charge in its molecule.
The elements or atoms present in non-polar solvents having electronegatives nearly same. Means there is very low electronegativity difference between atoms of non-polar solvents.
The non-polar solvents like aliphatic hydrocarbons includes alkanes i.e. hexane. In hexane esters can soluble. Similarly aromatic hydrocarbons like benzene in which esters can dissolve. Also other various non-polar solvents like chloroform, acetic acid, pyridine, etc. in which higher esters can dissolve.
Are esters soluble in water?
Yes, esters are soluble in water. The lower esters containing the hydrocarbon chain less than five carbon atoms are easily soluble in water. As the lower esters shows a polar nature. Water is also polar in nature. So, polar- polar molecules forms a bonding between each other.
As the hydrocarbon chain increases in ester molecules they become non-polar molecule and even can breaks the hydrogen bonds form between ester and water. The smaller ester molecules containing carbonyl (C=O) group having lone electron pairs and makes hydrogen bonds with either of the two hydrogen atoms of water molecules.
Are esters soluble in NaOH?
Yes, esters are soluble in base like sodium hydroxide (NaOH) or potassium hydroxide (KOH). A strong base sodium hydroxide (NaOH) which is used in the hydrolysis reactions of esters. There are two kind of ester hydrolysis reactions acid hydrolysis and base hydrolysis reactions of esters.
But esters are not easily soluble in strong base like NaOH, there is a need of some external energy to dissolve it like heat at high temperature, reflux, etc. The base hydrolysis of esters will form a carboxylate salt and R-OH molecule. In this reaction, carboxylic acid is formed as a final product as further the carboxylate salt reacts with strong acid like HCl.
Are esters soluble in hexane?
Yes, esters are soluble in hexane and also soluble in various organic solvent. Hexane is a long chain hydrocarbon compound comes under alkane category. Hexane has six carbon atoms in its molecule which is a non-polar solvent. The esters containing long chain of carbon atoms are also non-polar in nature, so they do dissolve in hexane.
As the long chain carbon containing esters are non-polar they can form bond with the non-polar hexane molecule and become soluble in it. The long chain carbon containing esters like methyl esters and long chain fatty acids, methyl palmitate, methyl sterate, methyl oleate, methyl linoleate, methyl petroselinate, etc. are the esters soluble in organic solvent like hexane.
Are esters soluble in oil?
Yes, esters are soluble in oils. Oils are naturally occurring esters which we are used in our daily routine from our diet to our hairs and body, we need oils. Many oils and fats we used in our daily routine are coming from natural sources like animals and plants.
In laboratory, oils and fats are produced by condensation reaction of carboxylic acids and glycerols (R-OH group). Oils which are liquids are room temperature contains large carbon- carbon double bonds as compared to fats which are solid at room temperature. As the higher esters containing more the five hydrocarbon chain can soluble in oils as the oils are already a form of an ester.
Are esters soluble in ammonia?
Yes, esters are soluble in ammonia. There is a reaction of ester with ammonia molecules which is known as ester ammonolysis. In this reaction the ester molecule get ammonolysed and the primary amide is produces as a final result.
In this chemical reaction ammonia is reacted with ester molecule which breaks the single covalent bond between carbon atom and oxygen atom (C-O) of ester molecule. So, from the ester ammonolysis reaction we can say that the esters can soluble in ammonia.
Are esters soluble in ether?
As we see the comparison of esters and ether, so the esters are polar in nature while ethers are both polar and non-polar in nature. Yet we had not seen often that the esters are directly soluble in ethers in any studies. But as the low carbon chain containing esters are polar in nature, so they should get soluble with polar ethers.
But we can reduce esters to ethers, i.e. ethers can be prepared by the reduction of esters. The reduction of esters is done by using the trimethyle silane and some metallic catalyst like titanium tetrachloride (TiCl4).
Are esters soluble in HCl?
Yes, esters can soluble in HCl by applying some energy during reaction like reflux, heat at high temperature, etc. The strong acids like hydrochloric acid (HCl) or sulphuric acid (H2SO4) are used specially in acid catalysed ester hydrolysis reactions. The esters acid catalysed hydrolysis gives carboxylic acids and R-OH group as final products. This reaction is generally carried out in reflux condition.
The acids like HCl (hydrochloric acid) and H2SO4 (sulphuric acid) are generally used in acid catalysed ester hydrolysis reactions. But these strong acids are used in aqueous forms. So, from all this above reasons we can say that esters are soluble in HCl.
Are esters more soluble than ketones?
No, esters are less soluble than ketones. As the esters shows the similar polar nature like aldehydes and ketones, still they are less soluble than ketone molecules. The ester molecules have weak dipole moment as compared to ketones and also esters are not able to form hydrogen bonds with each other.
Only lower esters are capable to produce hydrogen bonds with water molecules. Thus, the smaller esters i.e. low carbon chain containing esters have low boiling point than ketones. So, we can conclude that the esters are less soluble in nature than ketones.
- 11 Hydrophobic Examples: Facts You Should Know
- Hypotonic Solution: Definition,Examples,Principles,Effects.
- Exergonic vs Endergonic: Detailed Explanations And Insights
- Oganesson Chemical Properties (25 Facts You Should Know)
- 11 Constitutional Isomers Examples: With Detailed Facts
- Discover The 15 Incredible Facts On HNO3 + BeO Reaction