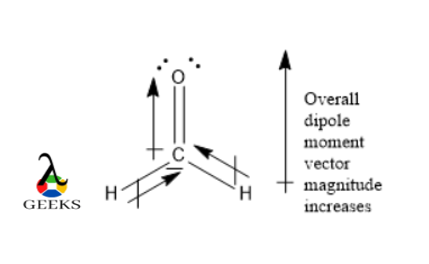
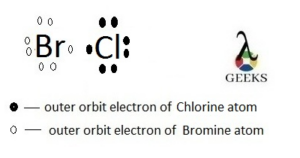
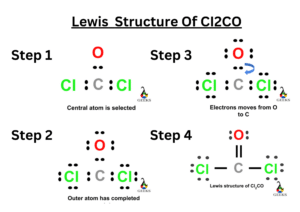
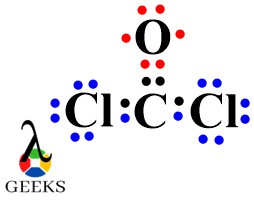
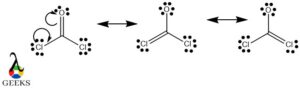
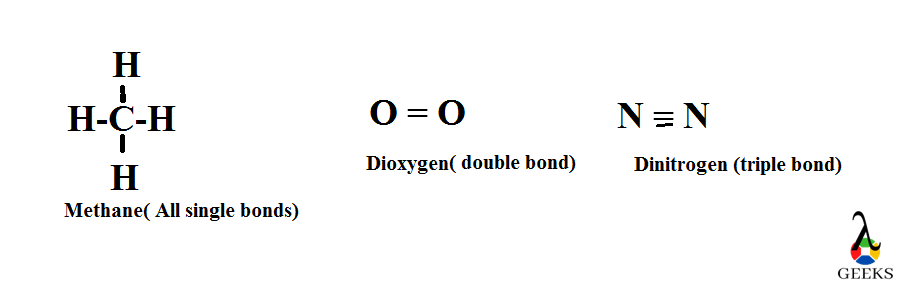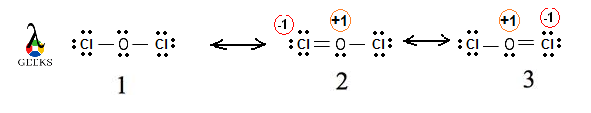The double bond Lewis structure is a representation of the arrangement of atoms and electrons in a molecule that contains a double bond. A double bond is formed when two atoms share two pairs of electrons. In a Lewis structure, the atoms are represented by their chemical symbols, and the shared electrons are shown as lines between the atoms. The double bond is represented by a double line. This structure helps us understand the bonding and geometry of molecules. It is an important concept in organic chemistry and plays a crucial role in determining the properties and reactivity of compounds.
Key Takeaways
| Double Bond Lewis Structure | |
|---|---|
| 1 | Represents the arrangement of atoms and electrons in a molecule with a double bond |
| 2 | Atoms are represented by chemical symbols |
| 3 | Shared electrons are shown as lines between atoms |
| 4 | Double bond is represented by a double line |
| 5 | Helps understand bonding and geometry of molecules |
| 6 | Important in organic chemistry |
| 7 | Determines properties and reactivity of compounds |
Double Bond Facts
Importance of Double Bonds in Lewis Structures
In chemical bonding, double bonds play a crucial role in determining the molecular structure and properties of compounds. Double bonds are a type of covalent bond formed when two pairs of electrons are shared between two atoms. These bonds are represented in Lewis structures, which are diagrams that show the arrangement of valence electrons in a molecule.
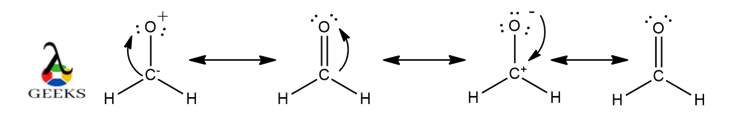
One of the key characteristics of double bonds is their ability to form resonance structures. Resonance occurs when there are multiple ways to arrange the double bonds within a molecule, resulting in different possible structures. This phenomenon is observed in molecules with alternating single and double bonds, such as benzene. Resonance structures contribute to the stability and unique properties of these compounds.
Double bonds also affect the molecular geometry of a molecule. According to the VSEPR (Valence Shell Electron Pair Repulsion) theory, electron pairs around an atom repel each other and arrange themselves to minimize repulsion. In molecules with double bonds, the presence of the double bond affects the arrangement of other atoms and electron pairs, leading to different molecular shapes.
Characteristics of Double Bonds
Double bonds have distinct characteristics that differentiate them from single bonds. These characteristics include bond length, bond energy, and bond type.
-
Bond Length: Double bonds are shorter than single bonds due to the increased electron density between the bonded atoms. The shorter bond length results in a stronger bond.
-
Bond Energy: Double bonds have higher bond energy than single bonds. This is because the sharing of two pairs of electrons in a double bond requires more energy to break compared to a single bond.
-
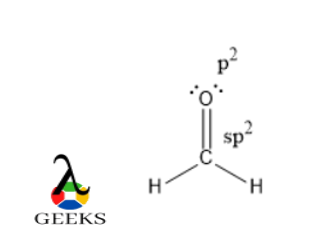
Bond Type: Double bonds consist of both sigma (σ) and pi (π) bonds. The sigma bond is formed by the overlap of atomic orbitals along the internuclear axis, while the pi bond is formed by the sideways overlap of p orbitals. The presence of pi bonds in double bonds contributes to the unique properties and reactivity of compounds.
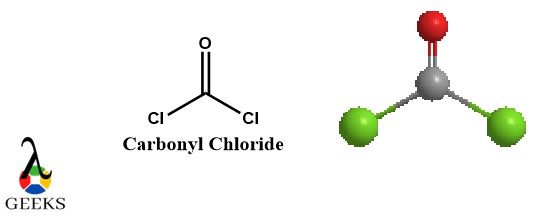
In organic chemistry, double bonds are commonly found in various chemical compounds, including alkenes and carbonyl compounds. These compounds play essential roles in many chemical reactions and are widely used in industries such as pharmaceuticals, polymers, and materials science.
Overall, understanding the importance and characteristics of double bonds is crucial for comprehending the molecular structure, reactivity, and properties of chemical compounds. Double bonds are fundamental building blocks in the field of chemistry and are extensively studied using theories such as Lewis theory and valence bond theory. The concept of double bonds also contributes to the understanding of molecular polarity, electronegativity, and chemical stability.
Types of Bonds in Lewis Structure

In chemical bonding, there are different types of bonds that contribute to the formation of molecular structures. These bonds are crucial in determining the properties and behavior of chemical compounds. The most common types of bonds in Lewis structures are single bonds, double bonds, and triple bonds.
Single Bonds
A single bond is a covalent bond formed when two atoms share one pair of electrons. It is represented by a single line (-) between the atoms in a Lewis dot diagram or structural formula. Single bonds are the most common type of bond in organic chemistry and play a vital role in the formation of various chemical compounds.
In a single bond, the atoms involved share two electrons, one from each atom’s valence shell. This sharing of electrons allows both atoms to achieve a stable electron configuration, following the octet rule. Single bonds are relatively weaker than double or triple bonds and have longer bond lengths.
Double Bonds
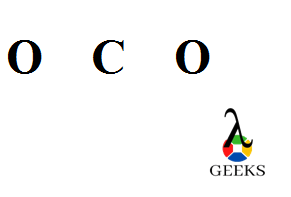
A double bond is a covalent bond formed when two atoms share two pairs of electrons. It is represented by a double line (=) between the atoms in a Lewis dot diagram or structural formula. Double bonds are commonly found in molecules with carbon-carbon or carbon-oxygen bonds, among others.
In a double bond, the atoms involved share four electrons, two from each atom’s valence shell. This sharing of electrons provides greater stability to the molecule and influences its molecular geometry. Double bonds are stronger and shorter than single bonds, contributing to the overall stability and reactivity of chemical compounds.
Triple Bonds
A triple bond is a covalent bond formed when two atoms share three pairs of electrons. It is represented by a triple line (≡) between the atoms in a Lewis dot diagram or structural formula. Triple bonds are less common than single or double bonds but are crucial in certain chemical reactions and compounds.
In a triple bond, the atoms involved share six electrons, three from each atom’s valence shell. This sharing of electrons results in a strong bond with a shorter bond length. Triple bonds are highly stable and require a significant amount of energy to break. They are often found in molecules with carbon-nitrogen or nitrogen-nitrogen bonds.
Understanding the different types of bonds in Lewis structures is essential for comprehending the molecular structure, chemical reactions, and properties of chemical compounds. These bonds, along with concepts like resonance structures, hybridization, and molecular orbitals, contribute to the overall stability, polarity, and reactivity of molecules.
Remember, the number of bonds formed between atoms depends on the number of valence electrons available and the octet rule. By sharing electrons, atoms can achieve a more stable electron configuration and form bonds that hold the molecule together.
Understanding Double Bonds in Lewis Structure
In chemical bonding, a double bond is a type of covalent bond that involves the sharing of two pairs of electrons between two atoms. It plays a crucial role in determining the molecular structure and properties of chemical compounds. Understanding how to identify, use, and determine double bonds in Lewis structures is essential in the field of organic chemistry and beyond.
How to Identify Double Bond in Lewis Structure

Identifying a double bond in a Lewis structure requires a good understanding of electron pairs and valence electrons. Here are a few key points to consider:
-
Resonance Structures: Double bonds often appear in resonance structures, which are different representations of the same molecule. Resonance structures show the delocalization of electrons and the presence of multiple bonding possibilities.
-
Octet Rule: The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight valence electrons. Double bonds can form when atoms share two pairs of electrons, allowing them to satisfy the octet rule.
-
Molecular Geometry: Double bonds can influence the molecular geometry of a molecule. They can cause atoms to be in a linear, trigonal planar, or tetrahedral arrangement, depending on the number and type of bonds present.
Lewis Structure Double Bond Rules
When drawing Lewis structures, certain rules guide the placement of double bonds:
-
Multiple Bond Priority: Double bonds take priority over single bonds when determining the placement of bonds in a Lewis structure. If there are multiple bonding possibilities, prioritize double bonds to achieve the most stable structure.
-
Hybridization: Double bonds often occur between atoms that have undergone hybridization. Hybrid orbitals allow for the formation of multiple bonds by providing the necessary electron density.
-
Pi Bond and Sigma Bond: A double bond consists of a sigma bond and a pi bond. The sigma bond is formed by the overlap of atomic orbitals, while the pi bond results from the sideways overlap of p orbitals. The pi bond is weaker and more reactive than the sigma bond.
When and How to Use Double Bond in Lewis Structure
Knowing when and how to use double bonds in Lewis structures is crucial for accurately representing chemical compounds. Here are a few guidelines:
-
Chemical Stability: Double bonds can increase the stability of a molecule by distributing electron density and reducing electron repulsion. They are commonly found in compounds that exhibit high chemical stability.
-
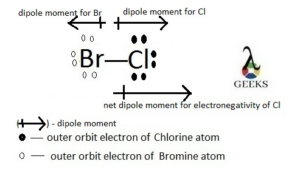
Molecular Polarity: Double bonds can contribute to the overall polarity of a molecule. The presence of double bonds can lead to regions of electron density imbalance, resulting in a polar molecule.
-
Electronegativity: Double bonds tend to form between atoms with significantly different electronegativities. The electronegativity difference creates a polar covalent bond, with one atom having a partial positive charge and the other having a partial negative charge.
How to Determine Double Bonds in Lewis Structures
Determining the number of double bonds in a Lewis structure requires careful consideration of the molecule’s electron configuration and bonding possibilities. Here’s how you can determine double bonds:
-
Valence Electrons: Determine the total number of valence electrons for the molecule by adding up the valence electrons of each atom.
-
Octet Rule: Distribute the valence electrons to satisfy the octet rule for each atom. Start by forming single bonds and then consider the remaining electrons for multiple bonding.
-
Lone Pair Electrons: If there are any remaining electrons after satisfying the octet rule, consider the possibility of forming double bonds. Lone pair electrons on an atom can be used to form double bonds with adjacent atoms.
By following these steps, you can accurately determine the presence and placement of double bonds in Lewis structures, providing a clear representation of the molecular structure.
Remember, understanding double bonds in Lewis structures is fundamental to comprehending chemical bonding, molecular geometry, and the behavior of chemical compounds in various reactions. It is a valuable tool in the field of organic chemistry and plays a significant role in the study of molecular models and structural formulas.
Practical Application of Double Bonds in Lewis Structure
Double bonds play a crucial role in the field of chemical bonding and molecular structure. They are a type of covalent bond that involves the sharing of two pairs of electrons between two atoms. Understanding the practical application of double bonds in Lewis structures is essential for predicting the behavior and properties of chemical compounds.
Examples of Compounds with Double Bonds
Double bonds can be found in a wide range of chemical compounds, both organic and inorganic. Some common examples include:
-
Ethene (C2H4): Ethene, also known as ethylene, is a hydrocarbon compound commonly used in the production of plastics and as a plant hormone. It consists of two carbon atoms connected by a double bond, with each carbon atom also bonded to two hydrogen atoms.
-
Carbon dioxide (CO2): Carbon dioxide is a greenhouse gas that is produced through various natural and human activities. It consists of one carbon atom bonded to two oxygen atoms through double bonds. The double bonds in carbon dioxide contribute to its stability and play a role in its reactivity in chemical reactions.
-
Nitrogen gas (N2): Nitrogen gas is a diatomic molecule that makes up a significant portion of Earth’s atmosphere. It consists of two nitrogen atoms connected by a triple bond, which is composed of one sigma bond and two pi bonds. The presence of double bonds in nitrogen gas contributes to its high stability and inertness.
Double Bond Lewis Structure Practice
To practice drawing Lewis structures for compounds with double bonds, it is important to understand the concept of valence electrons and the octet rule. Valence electrons are the electrons in the outermost energy level of an atom, and the octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight valence electrons.
Let’s take the example of ethene (C2H4) to practice drawing its Lewis structure. Carbon has four valence electrons, while hydrogen has one valence electron. The total number of valence electrons in ethene can be calculated as follows:
2 carbon atoms x 4 valence electrons = 8 valence electrons
4 hydrogen atoms x 1 valence electron = 4 valence electrons
The total number of valence electrons in ethene is 12. To draw the Lewis structure, we start by connecting the carbon atoms with a double bond. Each carbon atom is then bonded to two hydrogen atoms. The resulting Lewis structure for ethene is as follows:
H H
/
C=C
| |
H H
Double Bond Lewis Structure Examples
Here are a few more examples of Lewis structures for compounds with double bonds:
- Oxygen gas (O2): Oxygen gas consists of two oxygen atoms connected by a double bond. The Lewis structure for oxygen gas can be represented as follows:
O=O
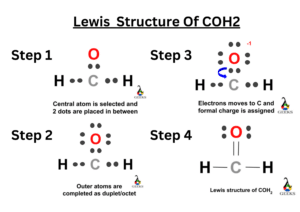
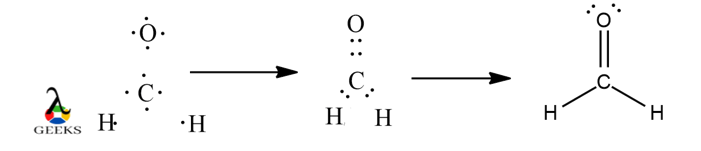
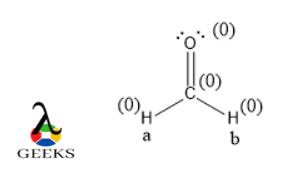
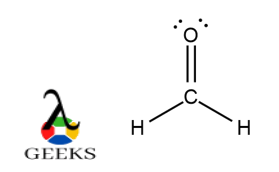
- Formaldehyde (CH2O): Formaldehyde is a compound used in various industrial applications. It consists of one carbon atom bonded to two hydrogen atoms and one oxygen atom through a double bond. The Lewis structure for formaldehyde can be represented as follows:
H
|
C=O
|
H
- Ethyne (C2H2): Ethyne, also known as acetylene, is a hydrocarbon compound commonly used in welding and as a fuel. It consists of two carbon atoms connected by a triple bond, which is composed of one sigma bond and two pi bonds. The Lewis structure for ethyne can be represented as follows:
H H
/
C≡C
By practicing drawing Lewis structures for compounds with double bonds, we can gain a better understanding of their molecular geometry, chemical stability, and reactivity. These structures provide valuable insights into the arrangement of atoms and electron pairs within a molecule, helping us predict and explain various chemical phenomena.
Remember, the examples provided here are just a starting point, and there are numerous other compounds with double bonds to explore in the fascinating world of chemistry.
Special Types of Double Bonds in Lewis Structure
Double Covalent Bond Lewis Structure
In chemical bonding, a double covalent bond is a special type of bond that involves the sharing of two pairs of electrons between two atoms. This type of bond is represented in the Lewis structure by a double line between the atoms. Double covalent bonds are commonly found in organic compounds and play a crucial role in determining the molecular structure and properties of these compounds.
When drawing the Lewis structure for a molecule with a double covalent bond, it is important to consider the valence electrons of the atoms involved. Valence electrons are the outermost electrons of an atom that participate in chemical bonding. In a double covalent bond, each atom contributes two electrons, resulting in a total of four electrons being shared between the two atoms.
To illustrate the double covalent bond Lewis structure, let’s take the example of ethene (C2H4). Ethene is a hydrocarbon molecule commonly known as ethylene, which is used in various industrial processes. The Lewis structure of ethene shows that each carbon atom forms a double bond with one of the hydrogen atoms, resulting in a linear molecular geometry.
Here is the Lewis structure of ethene:
H H
/
C=C
In this structure, the double bond between the carbon atoms is represented by two lines, indicating the sharing of two pairs of electrons. The remaining valence electrons are used to form single bonds with the hydrogen atoms.
Double and Triple Bonds Lewis Structure
Apart from double covalent bonds, there is another special type of bond known as a triple covalent bond. A triple covalent bond involves the sharing of three pairs of electrons between two atoms. This type of bond is represented in the Lewis structure by three lines between the atoms.
To understand the concept of double and triple bonds in Lewis structures, let’s consider the example of nitrogen gas (N2). Nitrogen gas is a diatomic molecule composed of two nitrogen atoms. Each nitrogen atom contributes three valence electrons, resulting in a total of six electrons being shared between the two atoms.
Here is the Lewis structure of nitrogen gas:
N≡N
In this structure, the triple bond between the nitrogen atoms is represented by three lines, indicating the sharing of three pairs of electrons. The remaining valence electrons are used to complete the octet rule for each nitrogen atom.
Double and triple bonds are important in chemical reactions as they influence the reactivity and stability of molecules. These types of bonds are also crucial in understanding the properties of chemical compounds and their behavior in various environments.
By incorporating double and triple bonds into Lewis structures, chemists can accurately represent the molecular geometry, electron distribution, and bonding patterns of different compounds. This information is essential for predicting the physical and chemical properties of substances and studying their reactivity.
Worksheet and Exercises
Welcome to the worksheet and exercises on double and triple bond Lewis structures! In this section, we will explore the fascinating world of chemical bonding and delve into the intricacies of covalent bonds and molecular structures. By understanding how electrons are shared between atoms, we can gain insights into the properties and behavior of various chemical compounds.
To begin, let’s review some key concepts related to chemical bonding. Atoms form covalent bonds by sharing electron pairs, specifically their valence electrons. These shared electron pairs are responsible for creating stable molecules with unique properties. Understanding how to represent these bonds using Lewis dot diagrams and structural formulas is crucial in organic chemistry.
One important aspect of covalent bonding is the presence of double and triple bonds. These bonds involve the sharing of two or three electron pairs, respectively, between two atoms. Double and triple bonds are often encountered in molecules containing carbon, such as hydrocarbons and organic compounds.
Now, let’s move on to the [‘Double and Triple Bond Lewis Structure Worksheet Answers‘] where we will apply our knowledge of chemical bonding and molecular structure.
[‘Double and Triple Bond Lewis Structure Worksheet Answers’]
In this section, we will work through a series of exercises to practice drawing Lewis structures for molecules with double and triple bonds. Remember to consider the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight valence electrons.
Here are some questions to test your understanding:
-
Draw the Lewis structure for ethene (C2H4), a molecule with a double bond between two carbon atoms. Indicate the hybridization of each carbon atom and the molecular geometry.
-
Determine the Lewis structure for acetylene (C2H2), a molecule with a triple bond between two carbon atoms. Identify the hybridization of each carbon atom and the molecular geometry.
-
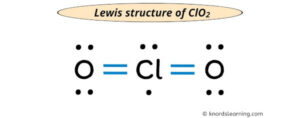
Draw the Lewis structure for nitrogen dioxide (NO2), a molecule with a double bond between nitrogen and one oxygen atom, and a single bond between nitrogen and the other oxygen atom. Identify the hybridization of the nitrogen atom and the molecular geometry.
-
Calculate the formal charge for each atom in the Lewis structure of carbon dioxide (CO2), a molecule with two double bonds between carbon and oxygen atoms.
Remember to consider resonance structures when applicable, as some molecules can have multiple valid Lewis structures. Resonance occurs when electron pairs can be delocalized, resulting in different arrangements of double and single bonds.
By practicing these exercises, you will enhance your understanding of molecular structures and gain proficiency in drawing Lewis structures for molecules with double and triple bonds. These skills are essential for predicting chemical reactions, understanding molecular polarity, and analyzing the properties of chemical compounds.
Keep exploring the fascinating world of chemical bonding and molecular structure, and enjoy your journey into the realm of covalent bonds and electron sharing!
References and Further Reading
Recommended Books and Articles on Lewis Structures and Double Bonds
If you’re looking to deepen your understanding of chemical bonding, covalent bonds, and molecular structure, there are several recommended books and articles that can provide valuable insights. These resources cover topics such as electron pairs, valence electrons, resonance structures, the octet rule, molecular geometry, and more. Here are some titles worth exploring:
-
“Organic Chemistry” by David R. Klein – This textbook is a great resource for learning about Lewis structures and their role in organic chemistry. It covers topics such as pi bonds, sigma bonds, the VSEPR theory, and chemical compounds. The book also includes numerous practice problems to reinforce your understanding.
In addition to these books, there are several articles available online that can further enhance your knowledge on Lewis structures and double bonds. Here are a few online resources worth checking out:
-
ChemGuide: Lewis Structures – This website provides a detailed explanation of Lewis structures, including their significance and how to draw them. It also covers topics such as chemical notation, structural formulas, and molecular models.
-
Khan Academy: Lewis Theory – Khan Academy offers a comprehensive video tutorial on Lewis theory, covering topics such as Lewis structures, valence bond theory, molecular polarity, and electronegativity. The tutorial includes interactive practice exercises to reinforce your understanding.
-
ChemLibreTexts: Lewis Structures and Bonding – This online resource provides a detailed explanation of Lewis structures and their role in chemical bonding. It also explores topics such as molecular geometry, resonance structures, and the octet rule.
Online Resources for Further Practice and Learning
To further practice and enhance your understanding of Lewis structures and double bonds, there are several online resources available. These resources offer interactive exercises, practice problems, and additional learning materials. Here are a few online platforms worth exploring:
-
Chemistry LibreTexts – Chemistry LibreTexts offers a wide range of courses and learning materials on various chemistry topics, including Lewis structures and double bonds. The platform provides interactive quizzes, practice problems, and comprehensive course materials to help you solidify your understanding.
-
ChemSpider – ChemSpider is a chemical structure database that allows you to search for and explore various chemical compounds. It can be a valuable resource for visualizing Lewis structures and exploring their properties.
-
ChemDoodle – ChemDoodle is a chemical drawing tool that allows you to create and manipulate Lewis structures and other molecular diagrams. It offers a user-friendly interface and various features to help you practice and visualize molecular structures.
These online resources provide valuable opportunities for further practice and learning, allowing you to deepen your understanding of Lewis structures and double bonds. Take advantage of these resources to reinforce your knowledge and enhance your skills in organic chemistry.
Frequently Asked Questions
When do you make a double bond in a Lewis structure?
A double bond in a Lewis structure is made when two atoms share two pairs of electrons. This typically occurs when both atoms involved have an incomplete octet of electrons and need to share more than one pair to achieve stability. It’s common in molecules such as oxygen (O2) and carbon dioxide (CO2).
When do you add a double bond in a Lewis structure?
A double bond is added in a Lewis structure when two atoms need to share two pairs of electrons to satisfy the octet rule. This rule states that atoms seek to have eight electrons in their outermost shell to achieve maximum stability. For example, in carbon dioxide (CO2), carbon forms double bonds with each oxygen atom.
How to know when to double bond in Lewis structure?
To know when to double bond in a Lewis structure, you need to count the total number of valence electrons for all atoms involved. If an atom does not have a complete octet after single bonds are formed, a double bond may be necessary. It’s also important to consider the concept of resonance, where a molecule can have multiple valid Lewis structures.
How to determine double bonds in Lewis structures?
Double bonds in Lewis structures are determined by the need for atoms to satisfy the octet rule. If an atom does not have a complete octet after single bonds are formed, a double bond may be necessary. Additionally, the concept of formal charge can be used to determine the most likely structure, with the structure having the smallest formal charges being the most likely.
When to use double bonds in Lewis structure?
Double bonds are used in Lewis structures when two atoms need to share two pairs of electrons to satisfy the octet rule. They are also used when the molecule exhibits resonance, meaning it can be represented by multiple valid Lewis structures.
What is a double bond in Lewis structure?
A double bond in a Lewis structure represents two shared pairs of electrons between two atoms. It consists of one sigma bond (σ) and one pi bond (π). The sigma bond is formed by the end-to-end overlapping of atomic orbitals, while the pi bond is formed by the side-to-side overlapping.
Can you provide a double bond Lewis structure example?
A common example of a double bond in a Lewis structure is carbon dioxide (CO2). In this molecule, the carbon atom forms double bonds with each of the two oxygen atoms. Each double bond consists of four shared electrons – two in a sigma bond and two in a pi bond.
Is a double bond always present in a Lewis structure?
A double bond is not always present in a Lewis structure. It only occurs when two atoms need to share two pairs of electrons to satisfy the octet rule or when the molecule exhibits resonance.
How to practice drawing double bond Lewis structures?
To practice drawing double bond Lewis structures, start with simple molecules like oxygen (O2) or carbon dioxide (CO2). Count the total number of valence electrons, then draw single bonds between atoms and distribute the remaining electrons. If any atoms lack a complete octet, consider adding a double bond.
What is a double covalent bond in a Lewis structure?
A double covalent bond in a Lewis structure represents the sharing of two pairs of electrons between two atoms. It’s depicted as two lines between the symbols of the atoms involved. This type of bond is common in many organic compounds and contributes to the stability and unique properties of these molecules.
Also Read: