Nitrogen dioxide (NO2) is a chemical compound composed of nitrogen and oxygen atoms. It is a reddish-brown gas with a pungent odor and is commonly found in urban areas as a result of air pollution. Understanding the Lewis structure of NO2 is important in determining its chemical properties and reactivity. The Lewis structure provides a visual representation of the arrangement of atoms and electrons in a molecule, helping us understand how the molecule interacts with other substances. In this article, we will explore the NO2 Lewis structure in detail, discussing its electron arrangement, bond formation, and overall molecular shape. So, let’s dive in and unravel the mysteries of NO2!
Key Takeaways
- The NO2 Lewis structure consists of a nitrogen atom bonded to two oxygen atoms.
- The nitrogen atom has a lone pair of electrons, while the oxygen atoms have three lone pairs each.
- The nitrogen-oxygen bonds are represented by single bonds, and the nitrogen-oxygen double bond is represented by a double bond.
- The formal charges on the atoms in the NO2 Lewis structure are: nitrogen (-1), one oxygen (+1), and the other oxygen (0).
- The NO2 molecule has a bent shape due to the repulsion between the lone pairs of electrons on the nitrogen and oxygen atoms.
NO2 Lewis Structure

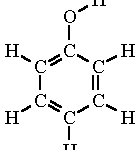
Drawing NO2 Lewis structure
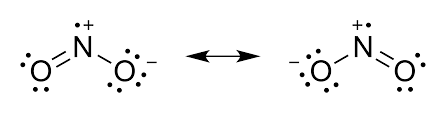
The Lewis structure is a way to represent the bonding and electron distribution in a molecule. In the case of NO2, which stands for nitrogen dioxide, we can draw its Lewis structure to understand its molecular geometry and electron arrangement.
To draw the Lewis structure of NO2, we need to follow a few steps:
-
Determine the total number of valence electrons: In NO2, nitrogen (N) is in Group 5A of the periodic table, so it has 5 valence electrons. Oxygen (O) is in Group 6A, so each oxygen atom has 6 valence electrons. Since there are two oxygen atoms in NO2, we have a total of 5 + 2(6) = 17 valence electrons.
-
Identify the central atom: In NO2, nitrogen is the central atom as it is less electronegative than oxygen.
-
Connect the atoms: Place the nitrogen atom in the center and connect it to the two oxygen atoms using single bonds.
-
Distribute the remaining electrons: Distribute the remaining electrons around the atoms to satisfy the octet rule. Start by placing lone pairs on the outer atoms (oxygen) and then distribute the remaining electrons on the central atom (nitrogen).
-
Check for octet rule and formal charges: Make sure all atoms have an octet of electrons (except hydrogen, which only needs 2 electrons). If necessary, move lone pairs to form double or triple bonds to satisfy the octet rule. Also, check for any formal charges to minimize their presence.
Explanation of NO2 Lewis structure
The Lewis structure of NO2 shows that nitrogen is bonded to two oxygen atoms. The nitrogen atom has one lone pair of electrons, while each oxygen atom has two lone pairs. The double bond between nitrogen and one oxygen atom is represented by two dots or a double line, indicating the sharing of two pairs of electrons. The single bond between nitrogen and the other oxygen atom is represented by a single dot or a single line, indicating the sharing of one pair of electrons.
The Lewis structure helps us understand the arrangement of electrons in a molecule and predict its molecular geometry. In the case of NO2, the molecule has a bent or V-shaped geometry due to the repulsion between the lone pairs of electrons on the oxygen atoms. This bent shape gives NO2 a polar nature, with the oxygen atoms being slightly negative and the nitrogen atom being slightly positive.
Valence electrons in NO2 Lewis structure
Valence electrons are the electrons in the outermost energy level of an atom. In the Lewis structure of NO2, we consider the valence electrons of nitrogen and oxygen to determine the total number of electrons available for bonding.
Nitrogen, being in Group 5A, has 5 valence electrons. Oxygen, being in Group 6A, has 6 valence electrons. Since there are two oxygen atoms in NO2, we multiply the number of valence electrons of oxygen by 2. Therefore, the total number of valence electrons in NO2 is 5 + 2(6) = 17.
Valence electrons play a crucial role in determining the chemical properties and reactivity of a molecule. They are involved in forming chemical bonds and determining the electron distribution in a molecule.
Octet rule in NO2 Lewis structure
The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with 8 electrons in their outermost energy level. This rule helps us understand the formation of chemical bonds and the stability of molecules.
In the Lewis structure of NO2, we can see that nitrogen has 5 valence electrons and each oxygen atom has 6 valence electrons. By sharing electrons through single and double bonds, nitrogen and oxygen can achieve an octet of electrons in their outermost energy level.
The double bond between nitrogen and one oxygen atom satisfies the octet rule for both nitrogen and oxygen. However, the other oxygen atom only has 7 electrons around it. To satisfy the octet rule, one lone pair from the nitrogen atom is moved to form a double bond with the second oxygen atom. This redistribution of electrons allows all atoms in NO2 to have an octet of electrons, fulfilling the octet rule.
Understanding the NO2 Lewis structure and the application of the octet rule helps us predict the stability and reactivity of molecules. It provides insights into the chemical behavior and properties of compounds like nitrogen dioxide.
Hybridization in NO2 Lewis Structure
The Lewis structure of NO2, also known as nitrogen dioxide, is a representation of the molecule’s bonding and electron arrangement. In order to understand the Lewis structure of NO2, it is important to first grasp the concept of hybridization.
Definition of Hybridization
Hybridization is a concept in chemistry that describes the mixing of atomic orbitals to form new hybrid orbitals. These hybrid orbitals have different shapes and energies compared to the original atomic orbitals. Hybridization occurs when atoms bond together to form molecules.
Hybridization in NO2
In the case of NO2, the central nitrogen atom is bonded to two oxygen atoms. To determine the hybridization of the nitrogen atom in NO2, we need to consider the number of electron groups around it. An electron group can be a lone pair or a bond.
In NO2, there are two oxygen atoms bonded to the nitrogen atom, resulting in two electron groups. Additionally, there is one lone pair of electrons on the nitrogen atom. Therefore, the total number of electron groups around the nitrogen atom is three.
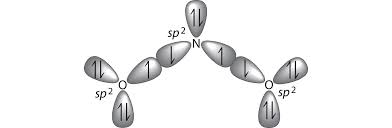
Explanation of sp2 Hybridization in NO2
The hybridization of the nitrogen atom in NO2 is sp2. This means that the nitrogen atom in NO2 undergoes hybridization by mixing one 2s orbital and two 2p orbitals to form three sp2 hybrid orbitals. These sp2 hybrid orbitals are arranged in a trigonal planar geometry around the nitrogen atom.
The three sp2 hybrid orbitals in NO2 are used to form sigma bonds with the two oxygen atoms and accommodate the lone pair of electrons. The remaining p orbital on the nitrogen atom contains one electron, which is involved in pi bonding with one of the oxygen atoms.
To summarize, the sp2 hybridization in NO2 allows the nitrogen atom to form three sigma bonds and one pi bond, resulting in a trigonal planar molecular geometry.
In conclusion, understanding the hybridization in the NO2 Lewis structure is crucial for comprehending the molecule’s bonding and shape. The sp2 hybridization of the nitrogen atom in NO2 enables it to form three sigma bonds and one pi bond, leading to a trigonal planar molecular geometry.
Formal Charges in NO2 Lewis Structure
The formal charges in a NO2 Lewis structure play a crucial role in understanding the distribution of electrons within the molecule. By assigning formal charges, we can determine the most stable arrangement of electrons and gain insights into the molecule’s reactivity and properties.
Definition of Formal Charges
Formal charges are hypothetical charges assigned to each atom in a molecule or ion. These charges help us understand the distribution of electrons and determine the stability of different resonance structures. The formal charge of an atom is calculated by comparing the number of valence electrons it should have with the number it actually possesses in the Lewis structure.
Calculation of Formal Charges in NO2
To calculate the formal charges in a NO2 molecule, we need to follow a step-by-step process:
-
Determine the total number of valence electrons in the molecule. For NO2, nitrogen (N) contributes 5 valence electrons, and each oxygen (O) contributes 6 valence electrons, giving us a total of 5 + 2(6) = 17 valence electrons.
-
Assign lone pairs of electrons to each atom. Nitrogen requires 3 lone pairs to complete its octet, while each oxygen atom requires 2 lone pairs.
-
Connect the atoms using single bonds. In the case of NO2, nitrogen forms a double bond with one oxygen atom, and a single bond with the other oxygen atom.
-
Distribute the remaining electrons as lone pairs to satisfy the octet rule for each atom. In NO2, the remaining 3 electrons are placed as a lone pair on the nitrogen atom.
-
Calculate the formal charge for each atom. The formula for formal charge is:
Formal Charge = Valence Electrons – Lone Pair Electrons – 0.5 * Bonding Electrons
For example, for the nitrogen atom in NO2, the formal charge is:
Formal Charge = 5 – 3 – 0.5 * 4 = 0
For each oxygen atom, the formal charge is:
Formal Charge = 6 – 2 – 0.5 * 4 = 0
Formal Charges in NO2 Lewis Structure
In the NO2 Lewis structure, the nitrogen atom has a formal charge of 0, while each oxygen atom also has a formal charge of 0. This distribution of formal charges indicates that the Lewis structure is stable and represents the most favorable arrangement of electrons for NO2.
By analyzing the formal charges, we can conclude that the nitrogen atom in NO2 does not carry any excess or deficient electrons. Similarly, each oxygen atom also has the appropriate number of electrons to maintain stability.
Understanding the formal charges in the NO2 Lewis structure provides valuable insights into the molecule’s behavior and reactivity. It helps us predict how NO2 interacts with other molecules and how it participates in chemical reactions.
In the next section, we will explore the molecular geometry and bond angles in the NO2 molecule, further enhancing our understanding of its structure and properties.
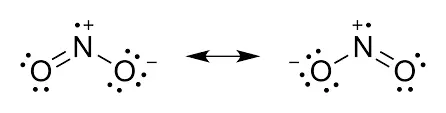
Resonance in NO2 Lewis Structure
Resonance is an important concept in chemistry that helps us understand the behavior of molecules. In the case of the NO2 Lewis structure, resonance plays a significant role in determining its structure and properties.
Definition of Resonance
Resonance refers to the delocalization of electrons within a molecule. It occurs when there are multiple valid Lewis structures that can be drawn for a molecule, and the actual structure is a combination or average of these resonance structures. In other words, resonance structures are different ways of arranging the same atoms, but with different electron distributions.
Resonance in NO2 Lewis Structure
When we consider the NO2 molecule, we can draw multiple resonance structures. NO2, also known as nitrogen dioxide, consists of a nitrogen atom bonded to two oxygen atoms. The central nitrogen atom has a lone pair of electrons and forms a double bond with one of the oxygen atoms, while the other oxygen atom is bonded by a single bond.
To represent the resonance in the NO2 Lewis structure, we can draw two resonance structures. In the first resonance structure, the double bond is between the nitrogen and the oxygen on the left, while in the second resonance structure, the double bond is between the nitrogen and the oxygen on the right. These resonance structures can be interconverted by moving the double bond and the lone pair of electrons.
Explanation of Resonating Structures in NO2
The presence of resonance in the NO2 Lewis structure affects the overall structure and properties of the molecule. Due to resonance, the actual structure of NO2 is a hybrid of the two resonance structures. This means that the double bond character is shared between the two oxygen atoms, resulting in a more stable molecule.
The resonance in NO2 also affects the bond lengths and bond angles within the molecule. In the resonance structures, the nitrogen-oxygen bond lengths are equal, and the nitrogen-oxygen-nitrogen bond angle is approximately 134 degrees. However, in the actual structure, the bond lengths are intermediate between single and double bonds, and the bond angle is slightly less than 134 degrees.
The presence of resonance also influences the polarity of the NO2 molecule. Each resonance structure has a partial positive charge on the nitrogen atom and a partial negative charge on the oxygen atoms. In the actual structure, the polarity is distributed over the molecule, resulting in a polar molecule.
In conclusion, resonance in the NO2 Lewis structure is a fascinating phenomenon that arises due to the delocalization of electrons. It leads to the formation of multiple resonance structures, which contribute to the overall stability, structure, and properties of the NO2 molecule. By understanding resonance, we can gain valuable insights into the behavior of molecules and their chemical reactions.
Bond Angle in NO2 Lewis Structure
The bond angle in the NO2 Lewis structure plays a crucial role in determining the shape and properties of the molecule. Understanding the bond angle is essential for predicting the molecule’s behavior and its interactions with other molecules. In this section, we will explore the definition of bond angle, discuss the bond angle in the NO2 Lewis structure, and explain the bent shape of NO2.
Definition of Bond Angle
The bond angle is the angle formed between two adjacent bonds in a molecule. It is measured in degrees and provides valuable information about the molecular geometry and the arrangement of atoms in a compound. The bond angle is influenced by various factors, including the number of electron pairs around the central atom and the repulsion between these electron pairs.
Bond Angle in NO2 Lewis Structure
To understand the bond angle in the NO2 Lewis structure, let’s first take a look at its molecular geometry. NO2, also known as nitrogen dioxide, consists of a central nitrogen atom bonded to two oxygen atoms. The Lewis structure for NO2 shows that nitrogen has one lone pair and two single bonds with oxygen.
In the NO2 Lewis structure, the central nitrogen atom is surrounded by three electron pairs – two from the oxygen atoms and one lone pair. These electron pairs repel each other, causing the molecule to adopt a bent shape. The bond angle in the NO2 Lewis structure is approximately 134 degrees.
Explanation of Bent Shape in NO2
The bent shape of NO2 can be explained by considering the repulsion between electron pairs. The lone pair on the central nitrogen atom exerts a greater repulsive force compared to the bonding pairs. As a result, the bonding pairs are pushed closer together, leading to a decrease in the bond angle.
The repulsion between the lone pair and the bonding pairs causes the NO2 molecule to bend, resulting in a bond angle less than the ideal 180 degrees. This bent shape is also influenced by the electronegativity difference between nitrogen and oxygen, which leads to a polar molecule.
In summary, the bond angle in the NO2 Lewis structure is approximately 134 degrees, indicating a bent shape. This bent shape is a result of the repulsion between the lone pair and the bonding pairs, as well as the electronegativity difference between nitrogen and oxygen. Understanding the bond angle and molecular geometry of NO2 is crucial for comprehending its chemical behavior and interactions with other substances.
Lone Pairs in NO2 Lewis Structure
In the Lewis structure of NO2, lone pairs play a crucial role in determining the molecule’s geometry and properties. Let’s explore the definition of lone pairs, the number of lone pairs in NO2, and the impact they have on the molecule’s geometry.
Definition of Lone Pairs
Lone pairs, also known as non-bonding pairs, are pairs of electrons that are not involved in bonding with other atoms. In a Lewis structure, these electrons are represented as dots around the atom. The presence of lone pairs affects the overall shape and polarity of a molecule.
Number of Lone Pairs in NO2
In the NO2 molecule, there are two oxygen atoms bonded to a central nitrogen atom. To determine the number of lone pairs in NO2, we need to consider the valence electrons of each atom. Nitrogen has five valence electrons, while oxygen has six. Therefore, the total number of valence electrons in NO2 is:
(1 × 5) + (2 × 6) = 17
To distribute these electrons, we first form single bonds between the nitrogen atom and each oxygen atom. This accounts for four electrons (two from each oxygen). The remaining 13 electrons are then placed as lone pairs around the atoms.
Impact of Lone Pairs on NO2 Geometry
The presence of lone pairs in NO2 affects its geometry and bond angles. In NO2, the nitrogen atom is surrounded by two oxygen atoms and one lone pair. This arrangement gives rise to a bent or V-shaped molecular geometry.
The repulsion between the lone pair and the bonding pairs causes a distortion in the molecule’s shape. The bond angle between the nitrogen-oxygen bonds is less than the ideal 120 degrees due to this repulsion. In the case of NO2, the bond angle is approximately 134 degrees.
The presence of lone pairs also influences the polarity of the molecule. The electronegativity of oxygen is higher than that of nitrogen, resulting in a polar covalent bond between nitrogen and oxygen. The lone pair on nitrogen further enhances the polarity of the molecule, making NO2 a polar molecule.
To summarize, the NO2 molecule has one lone pair on the central nitrogen atom, which affects its geometry, bond angle, and polarity. The presence of the lone pair causes a bent molecular shape and a bond angle of approximately 134 degrees. Additionally, the lone pair contributes to the overall polarity of the molecule.
In the next section, we will delve into the resonance structure of NO2 and its implications on the molecule’s stability and reactivity.
Polar or Nonpolar Nature of NO2 Lewis Structure
Definition of Polar and Nonpolar Molecules
Before we delve into the polar or nonpolar nature of the NO2 Lewis structure, let’s first understand what it means for a molecule to be polar or nonpolar.
In chemistry, polarity refers to the distribution of electrons in a molecule. A polar molecule has an uneven distribution of electron density, resulting in a separation of positive and negative charges. On the other hand, a nonpolar molecule has an even distribution of electron density, with no separation of charges.
The polarity of a molecule is determined by the difference in electronegativity between the atoms involved in the chemical bond. Electronegativity is a measure of an atom’s ability to attract electrons towards itself. When two atoms with different electronegativities form a bond, the more electronegative atom pulls the shared electrons closer to itself, creating a polar bond.
Determining Polarity of NO2 Lewis Structure
Now, let’s apply this knowledge to the NO2 Lewis structure to determine its polarity.
The NO2 molecule, also known as nitrogen dioxide, consists of one nitrogen atom (N) and two oxygen atoms (O). To draw the Lewis structure of NO2, we start by counting the total number of valence electrons in the molecule. Nitrogen contributes 5 valence electrons, while each oxygen contributes 6 valence electrons, giving us a total of 5 + 2(6) = 17 valence electrons.
Next, we arrange the atoms in the structure, placing the nitrogen atom in the center and the oxygen atoms on either side. We then connect the atoms using single bonds, which account for 2 electrons each. After connecting the atoms, we distribute the remaining electrons as lone pairs around the atoms to satisfy the octet rule.
In the NO2 Lewis structure, the nitrogen atom is double-bonded to one of the oxygen atoms, while the other oxygen atom has a lone pair. This arrangement gives nitrogen a formal charge of +1 and the oxygen atoms a formal charge of -1 each. The Lewis structure can be represented as follows:
O
╱
N = O
╲
O
Now, let’s analyze the polarity of the NO2 molecule based on its Lewis structure. The nitrogen-oxygen double bond is a polar bond due to the difference in electronegativity between nitrogen and oxygen. Oxygen is more electronegative than nitrogen, so it pulls the shared electrons closer to itself, creating a partial negative charge on the oxygen atom and a partial positive charge on the nitrogen atom.
Additionally, the lone pair on the oxygen atom also contributes to the polarity of the molecule. The presence of the lone pair creates an uneven distribution of electron density, further enhancing the polarity of the NO2 molecule.
Therefore, based on the arrangement of atoms and the polarity of the bonds and lone pairs, we can conclude that the NO2 molecule is polar in nature.
In summary, the NO2 Lewis structure exhibits polarity due to the polar nitrogen-oxygen double bond and the presence of a lone pair on one of the oxygen atoms. Understanding the polarity of molecules is crucial in various chemical reactions and interactions, as it influences the behavior and properties of substances.
VSEPR Model and NO2 Lewis Structure
The VSEPR (Valence Shell Electron Pair Repulsion) model is a useful tool in predicting the shape and geometry of molecules. By considering the repulsion between electron pairs, we can determine the arrangement of atoms in a molecule. In this section, we will explore the application of the VSEPR model to the NO2 Lewis structure and discuss the electron geometry of NO2.
Overview of VSEPR Model
The VSEPR model is based on the principle that electron pairs in the valence shell of an atom repel each other. This repulsion leads to a specific arrangement of atoms in a molecule, which determines its shape and geometry. The VSEPR model is widely used to predict molecular geometries and understand the behavior of molecules.
To apply the VSEPR model, we start by drawing the Lewis structure of the molecule. The Lewis structure shows the arrangement of atoms and valence electrons in a molecule. By counting the number of valence electrons and considering the octet rule, we can determine the Lewis structure of a molecule.
Application of VSEPR Model to NO2 Lewis Structure
Now let’s apply the VSEPR model to the NO2 molecule. NO2, also known as nitrogen dioxide, consists of one nitrogen atom (N) and two oxygen atoms (O).
To determine the Lewis structure of NO2, we first calculate the total number of valence electrons. Nitrogen has 5 valence electrons, and each oxygen atom has 6 valence electrons. Therefore, the total number of valence electrons in NO2 is:
5 (from nitrogen) + 2 * 6 (from oxygen) = 17
Next, we arrange the atoms in the molecule and connect them with single bonds. In the case of NO2, nitrogen is the central atom, and the two oxygen atoms are bonded to it.
To distribute the valence electrons, we place them around the atoms, starting with the outer atoms and then the central atom. In NO2, each oxygen atom needs 2 electrons to complete its octet, while nitrogen needs 3 electrons. This leaves us with 17 – 4 = 13 electrons to distribute.
We place the remaining electrons as lone pairs on the oxygen atoms. Each oxygen atom will have one lone pair, and nitrogen will have one lone pair as well.
Electron Geometry of NO2
The electron geometry of a molecule is determined by the arrangement of electron pairs around the central atom. In the case of NO2, nitrogen is the central atom, and it has one lone pair and two bonding pairs.
According to the VSEPR model, the presence of one lone pair and two bonding pairs gives NO2 an electron pair geometry of trigonal planar. This means that the electron pairs are arranged in a flat, triangular shape around the nitrogen atom.
The bond angle in NO2 is approximately 134 degrees. This angle is slightly less than the ideal bond angle of 120 degrees due to the repulsion between the lone pair and the bonding pairs.
In summary, the VSEPR model can be used to determine the electron geometry of NO2, which is trigonal planar. The presence of one lone pair and two bonding pairs results in a bond angle of approximately 134 degrees. Understanding the electron geometry of molecules like NO2 is crucial in predicting their physical and chemical properties.
Uses of NO2
Nitrogen dioxide (NO2) is a highly reactive and toxic gas that is commonly used in various industrial processes and applications. Its unique properties make it valuable for a range of purposes. Let’s explore some of the key uses of NO2.
Industrial production of Nitric acid
One of the primary uses of NO2 is in the industrial production of nitric acid. Nitric acid is a vital chemical compound used in the manufacturing of fertilizers, explosives, dyes, and pharmaceuticals. NO2 is a key intermediate in the Ostwald process, which involves the oxidation of ammonia to produce nitric acid. In this process, NO2 reacts with water to form nitric acid and nitrogen monoxide (NO). The production of nitric acid is crucial for various industries, making NO2 an essential component in its synthesis.
Catalyst in chemical reactions
NO2 also serves as a catalyst in several chemical reactions. A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. NO2 acts as a catalyst in the oxidation of sulfur dioxide (SO2) to form sulfur trioxide (SO3). This reaction is a crucial step in the production of sulfuric acid, which is widely used in the manufacturing of fertilizers, detergents, and other chemical processes. The presence of NO2 enhances the efficiency of the reaction, making it an important catalyst in the sulfuric acid production process.
Regulation of sulfuric acid production
In addition to its role as a catalyst, NO2 also plays a significant role in the regulation of sulfuric acid production. The concentration of NO2 in the reaction mixture affects the rate of the oxidation reaction. By controlling the amount of NO2 present, manufacturers can regulate the production of sulfuric acid and ensure optimal efficiency. This regulation is crucial for maintaining the quality and quantity of sulfuric acid produced, as well as minimizing the environmental impact of the process.
Use as an oxidizer in rocket fuels
NO2 finds application as an oxidizer in rocket fuels. In rocket propulsion systems, an oxidizer is required to support the combustion of the fuel. NO2 is a powerful oxidizer that provides the necessary oxygen for the combustion process. It is commonly used in combination with fuels such as hydrazine to create highly energetic propulsion systems. The use of NO2 as an oxidizer allows rockets to achieve high speeds and propel payloads into space.
Manufacture of oxidized cellulose compounds
NO2 is also utilized in the manufacture of oxidized cellulose compounds. Oxidized cellulose is a material derived from cellulose fibers that have been chemically modified to enhance their properties. NO2 is used in the oxidation process, which introduces functional groups onto the cellulose structure, resulting in improved strength, stability, and absorbency. Oxidized cellulose compounds find applications in various industries, including healthcare, textiles, and paper manufacturing.
In conclusion, NO2 has a wide range of uses in various industries and applications. From its role in the production of nitric acid and regulation of sulfuric acid production to its use as a catalyst and oxidizer, NO2 plays a crucial role in numerous chemical processes. Its unique properties make it a valuable resource for enhancing the efficiency and performance of various industrial applications.
Conclusion
In conclusion, understanding the NO2 Lewis structure is crucial for comprehending the chemical properties and behavior of nitrogen dioxide. By examining the arrangement of atoms and electrons within the molecule, we can gain insights into its polarity, reactivity, and overall stability. The Lewis structure of NO2 reveals that it consists of a central nitrogen atom bonded to two oxygen atoms, with one of the oxygen atoms carrying an unshared electron pair. This arrangement gives rise to a bent molecular geometry, resulting in a polar molecule with a partial positive charge on the nitrogen atom and partial negative charges on the oxygen atoms. The presence of a lone pair on one of the oxygen atoms makes NO2 highly reactive, particularly in terms of its involvement in atmospheric chemistry and air pollution. By studying the NO2 Lewis structure, scientists can better understand the behavior of this important compound and its impact on the environment and human health.
Frequently Asked Questions
1. How do you determine the Lewis structure of NO2?
To determine the Lewis structure of NO2 (nitrogen dioxide), you need to count the valence electrons and follow the octet rule. The Lewis structure for NO2 consists of a nitrogen atom bonded to two oxygen atoms, with a double bond between nitrogen and one oxygen atom, and a single bond between nitrogen and the other oxygen atom.
2. What is the hybridization of NO2?
The hybridization of NO2 (nitrogen dioxide) is sp2. In the Lewis structure of NO2, the nitrogen atom forms three sigma bonds with the two oxygen atoms and has one lone pair. This arrangement requires the nitrogen atom to undergo sp2 hybridization.
3. How many valence electrons are in the Lewis structure of NO2-?
In the Lewis structure of NO2- (nitrite ion), there are 18 valence electrons. The nitrogen atom contributes 5 valence electrons, and each oxygen atom contributes 6 valence electrons. The negative charge on the nitrite ion adds an additional electron, totaling 18 valence electrons.
4. Does the Lewis structure of NO2 follow the octet rule?
Yes, the Lewis structure of NO2 (nitrogen dioxide) follows the octet rule. The nitrogen atom has a double bond with one oxygen atom and a single bond with the other oxygen atom, resulting in a total of 8 valence electrons around the nitrogen atom.
5. Why does NO2 have a double bond?
NO2 (nitrogen dioxide) has a double bond because it allows the nitrogen atom to achieve a stable octet configuration. By forming a double bond with one of the oxygen atoms, the nitrogen atom can share two pairs of electrons, satisfying the octet rule.
6. What is the bond order in the Lewis structure of NO2+?
The bond order in the Lewis structure of NO2+ (nitronium ion) is 2. The nitrogen atom forms a double bond with one of the oxygen atoms and a coordinate covalent bond with the other oxygen atom, resulting in a bond order of 2.
7. How do you draw the Lewis structure of NO2?
To draw the Lewis structure of NO2 (nitrogen dioxide), start by placing the nitrogen atom in the center. Connect the nitrogen atom to two oxygen atoms using single bonds. Then, add a double bond between the nitrogen atom and one of the oxygen atoms. Finally, distribute any remaining valence electrons as lone pairs.
8. Does the Lewis structure of NO2 exhibit resonance?
Yes, the Lewis structure of NO2 (nitrogen dioxide) exhibits resonance. The double bond in the structure can be delocalized between the nitrogen atom and either of the oxygen atoms, resulting in resonance structures.
9. What is the bond angle in the Lewis structure of NO2?
The bond angle in the Lewis structure of NO2 (nitrogen dioxide) is approximately 134 degrees. The oxygen atoms are arranged in a bent shape around the nitrogen atom, resulting in a bond angle slightly less than 180 degrees.
10. Is the Lewis structure of NO2 polar or nonpolar?
The Lewis structure of NO2 (nitrogen dioxide) is polar. The presence of a bent molecular geometry and the unequal distribution of electrons due to the double bond result in a polar molecule.
Also Read: