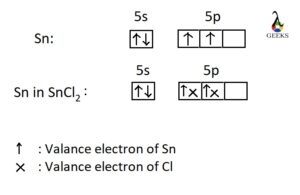
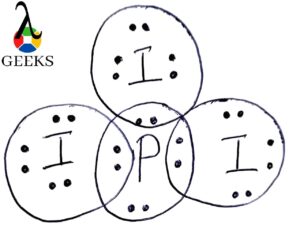
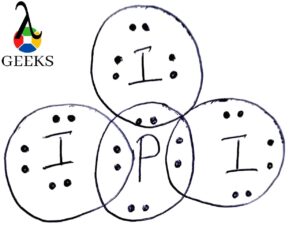
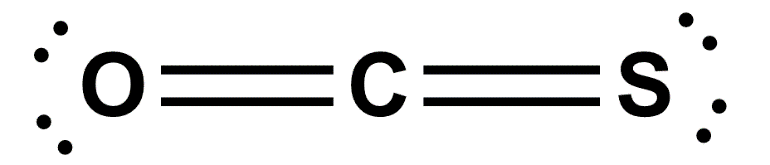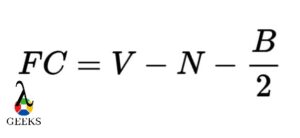The SEO2 Lewis structure refers to the representation of the molecule selenium dioxide (SeO2) using Lewis dot symbols. This structure helps us understand the arrangement of atoms and the distribution of electrons in the molecule. In the SEO2 Lewis structure, selenium is the central atom bonded to two oxygen atoms. Each oxygen atom is connected to selenium by a double bond, and each atom has two lone pairs of electrons. This arrangement gives SEO2 a bent molecular geometry. Understanding the SEO2 Lewis structure is important in studying the chemical properties and reactions of selenium dioxide.
Key Takeaways
| Atom | Number of Bonds | Number of Lone Pairs |
|---|---|---|
| Selenium | 2 | 2 |
| Oxygen | 2 | 2 |
Understanding the Basics of Lewis Structures
Definition and Importance of Lewis Structures
Lewis structures are a visual representation of the arrangement of atoms and electrons in a molecule. They provide valuable insights into the bonding and structure of molecules, helping us understand their properties and behavior. By using Lewis structures, we can determine the number of valence electrons in a molecule and predict its molecular geometry, bond angles, and polarity.
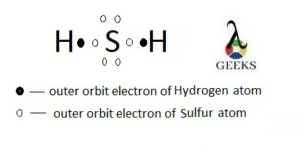
In order to draw a Lewis structure, we need to know the number of valence electrons each atom contributes to the molecule. Valence electrons are the outermost electrons of an atom and play a crucial role in chemical bonding. For example, let’s consider the Lewis dot structure of SEO2 (sulfur dioxide).
To find the Lewis structure of SEO2, we start by determining the number of valence electrons for each atom. Sulfur (S) is in Group 6A, so it has 6 valence electrons. Oxygen (O) is in Group 6A as well, so each oxygen atom contributes 6 valence electrons. Since there are two oxygen atoms in SEO2, the total number of valence electrons is 6 (from sulfur) + 6 (from oxygen) + 6 (from oxygen) = 18 valence electrons.
Next, we arrange the atoms in the molecule. In SEO2, sulfur is the central atom, and the two oxygen atoms are bonded to it. We represent the atoms using their chemical symbols (S and O) and connect them with lines to represent the bonds. In this case, sulfur forms a double bond with one oxygen atom and a single bond with the other oxygen atom.
To distribute the remaining valence electrons, we place them around the atoms in pairs, following the octet rule. The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable configuration with 8 valence electrons. In SEO2, after distributing the valence electrons, we find that sulfur has 8 electrons around it, while each oxygen atom has 8 electrons as well.
How to Find a Lewis Structure
Finding a Lewis structure involves a step-by-step process that can be summarized as follows:
- Determine the number of valence electrons for each atom in the molecule.
- Identify the central atom, which is usually the least electronegative atom or the one with the highest valence.
- Connect the atoms with single, double, or triple bonds, depending on the number of electrons needed to complete the octet rule.
- Distribute the remaining valence electrons around the atoms, ensuring that each atom has 8 electrons (except for hydrogen, which only needs 2 electrons).
- If there are still remaining valence electrons, place them as lone pairs on the central atom or atoms.
- Check if all atoms have achieved an octet or duet (for hydrogen). If not, try different arrangements or multiple bonds to achieve stability.
- Determine the molecular geometry and bond angles based on the arrangement of atoms and lone pairs.
- Assess the polarity of the molecule by considering the electronegativity difference between atoms and the molecular geometry.
It’s important to note that some molecules may have multiple valid Lewis structures, known as resonance structures. These structures differ only in the arrangement of electrons, not the connectivity of atoms. Resonance structures contribute to the overall stability of the molecule.
Understanding Lewis structures is fundamental in chemistry as it helps us predict the behavior of molecules and their interactions. By analyzing the arrangement of atoms and electrons, we can gain insights into the chemical bonding, molecular shape, and polarity of a compound.
The SeO2 Lewis Structure
How to Draw the SeO2 Lewis Structure
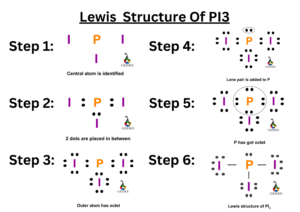
The SeO2 Lewis structure represents the arrangement of atoms and valence electrons in a molecule of selenium dioxide (SeO2). To draw the Lewis structure of SeO2, we need to follow a few steps:
-
Determine the total number of valence electrons in SeO2. Selenium (Se) belongs to Group 16, so it has 6 valence electrons, and each oxygen (O) atom has 6 valence electrons as well. Therefore, the total number of valence electrons in SeO2 is 6 + 2(6) = 18.
-
Identify the central atom. In SeO2, selenium (Se) is the central atom as it is less electronegative than oxygen (O).
-
Connect the atoms. Place the oxygen atoms around the selenium atom, forming single bonds between them. This will account for 4 of the 18 valence electrons.
-
Distribute the remaining valence electrons. Place the remaining 14 valence electrons as lone pairs on the oxygen atoms. Each oxygen atom should have 3 lone pairs.
-
Check for octet rule fulfillment. Count the total number of valence electrons used. If it is equal to the total number of valence electrons in SeO2 (18), then the octet rule is satisfied.
-
Determine the formal charges. Calculate the formal charges of each atom by subtracting the number of lone pair electrons and half the number of shared electrons from the number of valence electrons. The formal charge of an atom should ideally be as close to zero as possible.
-
Draw the Lewis structure. Represent the atoms using their symbols and connect them with lines to represent bonds. Place the lone pairs around the oxygen atoms.
The Lewis dot structure of SeO2 is as follows:
O
/
Se - O
O
Identifying the Correct Lewis Structure for SeO2
To identify the correct Lewis structure for SeO2, we need to consider the octet rule, formal charges, and the electronegativity of the atoms involved. The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with a full outer shell of electrons.
In SeO2, the selenium atom has 6 valence electrons and forms two single bonds with oxygen atoms, leaving it with a total of 4 valence electrons. Each oxygen atom has 6 valence electrons and forms one bond with selenium, leaving them with 5 valence electrons each. The formal charges of the atoms in SeO2 are as follows:
- Selenium (Se): 6 – 0.5(4) – 0 = 4
- Oxygen (O): 6 – 0.5(2) – 0 = 5
The Lewis structure of SeO2 satisfies the octet rule for all atoms and minimizes formal charges, making it the correct structure.
SeO2 Lewis Dot Structure
The Lewis dot structure of SeO2 shows the arrangement of atoms and valence electrons in the molecule. In SeO2, the selenium atom is surrounded by two oxygen atoms, with each oxygen atom forming a single bond with selenium. The remaining valence electrons are placed as lone pairs on the oxygen atoms.
The SeO2 molecule has a bent molecular geometry, with bond angles of approximately 119 degrees. This bent shape is a result of the lone pairs on the oxygen atoms, which repel the bonding pairs and cause the molecule to adopt a bent structure.
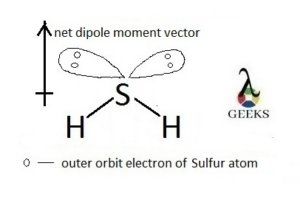
SeO2 is a polar molecule due to the difference in electronegativity between selenium and oxygen. The oxygen atoms are more electronegative than selenium, resulting in a partial negative charge on the oxygen atoms and a partial positive charge on the selenium atom.
In terms of hybridization, the selenium atom in SeO2 undergoes sp2 hybridization. This means that the selenium atom forms three sigma bonds with the oxygen atoms, using two of its p orbitals and one of its s orbitals.
Overall, understanding the SeO2 Lewis structure helps us comprehend the chemical bonding, electron pair geometry, molecular shape, and polarity of the molecule. It is an essential concept in chemistry that accounts for the advanced understanding of social and environmental interactions involving selenium compounds.
For more information on the SeO2 Lewis structure, you can visit the following link: awonline.sdsu.edu.
Key Aspects of SeO2 Lewis Structure
The Lewis structure of SeO2, also known as selenium dioxide, is an important concept in chemistry. It helps us understand the arrangement of atoms and electrons in this molecule. Let’s explore some key aspects of the SeO2 Lewis structure.
SeO2 Lewis Structure Lone Pair

In the SeO2 molecule, selenium (Se) is the central atom, while the two oxygen (O) atoms are bonded to it. Selenium has six valence electrons, and each oxygen atom contributes six valence electrons as well. This gives us a total of 18 valence electrons for SeO2.
When we draw the Lewis structure of SeO2, we find that there is one lone pair of electrons on the selenium atom. This lone pair is not involved in any bonding and is represented as a pair of dots next to the selenium atom.
SeO2 Lewis Structure Octet Rule

The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with eight valence electrons. In the case of SeO2, both oxygen atoms form double bonds with the selenium atom, sharing two pairs of electrons each.
By forming these double bonds, each oxygen atom achieves an octet of electrons, while the selenium atom has a total of 12 valence electrons around it, including the lone pair. Although the selenium atom does not have a complete octet, it is still stable due to its expanded valence shell.
SeO2 Lewis Structure Formal Charge
Formal charge is a concept used to determine the distribution of electrons in a molecule. It helps us understand the stability and reactivity of different atoms within a compound. To calculate the formal charge of an atom, we compare the number of valence electrons it should have with the number it actually has in the Lewis structure.
In the SeO2 Lewis structure, the formal charge of the selenium atom is zero, as it has six valence electrons and is surrounded by 12 electrons (including the lone pair). Each oxygen atom has a formal charge of zero as well, as they each have six valence electrons and are surrounded by eight electrons.
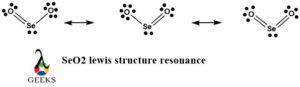
SeO2 Lewis Structure Resonance

Resonance occurs when there are multiple valid Lewis structures that can be drawn for a molecule. In the case of SeO2, resonance is not observed because there is only one way to arrange the atoms and electrons that satisfies the octet rule and minimizes formal charges.
SeO2 Lewis Structure Hybridization
Hybridization refers to the mixing of atomic orbitals to form new hybrid orbitals that are used for bonding. In SeO2, the selenium atom is sp2 hybridized, meaning that it forms three hybrid orbitals by mixing one s orbital and two p orbitals. These hybrid orbitals then overlap with the p orbitals of the oxygen atoms to form the sigma bonds in the molecule.
SeO2 Lewis Structure Molecular Geometry
The molecular geometry of SeO2 is bent or V-shaped. This shape is a result of the two oxygen atoms being bonded to the selenium atom and the presence of the lone pair on the selenium atom. The bond angles in SeO2 are approximately 119 degrees.
Polarity of SeO2 Lewis Structure
SeO2 Lewis Structure Polarity
The polarity of a molecule is determined by the presence of polar bonds and the molecular geometry. In the case of SeO2 (selenium dioxide), the Lewis dot structure can help us understand its polarity.
To determine the Lewis dot structure of SeO2, we need to know the number of valence electrons in SeO2. Selenium (Se) is in Group 16 of the periodic table and has 6 valence electrons, while each oxygen (O) atom has 6 valence electrons. Therefore, the total number of valence electrons in SeO2 is 6 + 2(6) = 18.
To draw the Lewis dot structure of SeO2, we start by placing the selenium atom in the center and connecting it to the two oxygen atoms with single bonds. Each oxygen atom will have three lone pairs of electrons around it. The remaining two valence electrons will be placed on the selenium atom.
The Lewis dot structure of SeO2 can be represented as:
O
//
Se = O //
\
O
Now, let’s analyze the polarity of SeO2. In a polar molecule, there is an uneven distribution of electron density, resulting in a partial positive and partial negative charge. This occurs when there is a difference in electronegativity between the atoms involved in the bond.
In SeO2, the oxygen atoms are more electronegative than the selenium atom. Oxygen has an electronegativity value of 3.44, while selenium has an electronegativity value of 2.55. This difference in electronegativity creates polar bonds between selenium and oxygen.
Is SeO2 Polar or Nonpolar?
Based on the Lewis dot structure and the presence of polar bonds, we can determine the overall polarity of SeO2. In SeO2, the oxygen atoms are pulling the electron density towards themselves, creating a partial negative charge on the oxygen atoms and a partial positive charge on the selenium atom.
Therefore, SeO2 is a polar molecule due to the unequal distribution of electron density caused by the polar bonds and the bent molecular geometry.
Why is SeO2 Polar?
The polarity of SeO2 can be explained by considering the molecular geometry and the presence of lone pairs of electrons. In SeO2, the molecule adopts a bent or V-shaped geometry due to the repulsion between the lone pairs of electrons on the oxygen atoms.
The presence of lone pairs on the oxygen atoms causes the bond angles to deviate from the ideal 180 degrees, resulting in a bent molecular shape. This bent shape leads to an uneven distribution of electron density, making SeO2 a polar molecule.
Additional Characteristics of SeO2
Explain the Solubility of the SeO2 Lewis Structure
The solubility of the SeO2 Lewis structure is an important characteristic to consider. SeO2 is a polar molecule due to the difference in electronegativity between selenium (Se) and oxygen (O) atoms. The oxygen atoms are more electronegative than selenium, causing a partial negative charge on the oxygen atoms and a partial positive charge on the selenium atom. This polarity allows SeO2 to dissolve in polar solvents such as water. When SeO2 is added to water, the polar water molecules interact with the polar SeO2 molecule, resulting in the dissolution of SeO2.
Describe the Toxicity of SeO2
SeO2 is known to be toxic and can pose health risks if not handled properly. Exposure to SeO2 can occur through inhalation, ingestion, or skin contact. The toxicity of SeO2 is primarily due to its ability to react with biological molecules, disrupting cellular processes. It can cause irritation to the respiratory system, eyes, and skin. Prolonged exposure to high concentrations of SeO2 can lead to more severe health effects, including respiratory issues, lung damage, and even death. Therefore, it is important to handle SeO2 with caution and follow proper safety protocols.
Is SeO2 Linear?
The molecular geometry of SeO2 determines its shape and whether it is linear or not. In the case of SeO2, it has a bent or V-shaped molecular geometry. This means that the molecule is not linear. The central selenium atom is surrounded by two oxygen atoms, forming a bent shape. The bond angles in SeO2 are approximately 119 degrees, which deviates from the ideal bond angle of 120 degrees for a trigonal planar arrangement. The presence of lone pairs on the oxygen atoms contributes to the bent shape of SeO2.
Comparing SeO2 Lewis Structure with Other Selenium Oxides
Lewis Structure of SeO
The Lewis structure of SeO (selenium oxide) consists of one selenium atom bonded to one oxygen atom. Selenium has 6 valence electrons, while oxygen has 6 valence electrons. To satisfy the octet rule, selenium shares two electrons with oxygen, forming a double bond. The Lewis dot structure of SeO can be represented as Se=O.
Lewis Structure of SeO3
The Lewis structure of SeO3 (selenium trioxide) involves one selenium atom bonded to three oxygen atoms. Selenium has 6 valence electrons, and each oxygen atom has 6 valence electrons. To fulfill the octet rule, selenium forms three single bonds with the oxygen atoms. The Lewis dot structure of SeO3 can be depicted as Se-O | O | O.
Lewis Structure of SeO4 2-
The Lewis structure of SeO4 2- (selenate ion) consists of one selenium atom bonded to four oxygen atoms. Selenium has 6 valence electrons, and each oxygen atom has 6 valence electrons. To satisfy the octet rule, selenium forms four single bonds with the oxygen atoms. Additionally, the ion carries a 2- charge, indicating the addition of two extra electrons. The Lewis dot structure of SeO4 2- can be represented as Se-O | O | O | O with two extra electrons.
SeO3-2 Lewis Structure
The Lewis structure of SeO3-2 (selenite ion) involves one selenium atom bonded to three oxygen atoms. Selenium has 6 valence electrons, and each oxygen atom has 6 valence electrons. To fulfill the octet rule, selenium forms three single bonds with the oxygen atoms. Furthermore, the ion carries a 2- charge, indicating the addition of two extra electrons. The Lewis dot structure of SeO3-2 can be depicted as Se-O | O | O with two extra electrons.
SeO2 2- Lewis Structure
The Lewis structure of SeO2 2- (diselenite ion) consists of one selenium atom bonded to two oxygen atoms. Selenium has 6 valence electrons, and each oxygen atom has 6 valence electrons. To satisfy the octet rule, selenium forms two single bonds with the oxygen atoms. Additionally, the ion carries a 2- charge, indicating the addition of two extra electrons. The Lewis dot structure of SeO2 2- can be represented as Se-O | O with two extra electrons.
When comparing the Lewis structure of SeO2 with other selenium oxides, we can observe the following differences:
- SeO2 has one selenium atom bonded to two oxygen atoms, while SeO3, SeO4 2-, SeO3-2, and SeO2 2- have one selenium atom bonded to three or four oxygen atoms.
- SeO2 has a double bond between selenium and oxygen, while the other selenium oxides have single bonds.
- SeO4 2-, SeO3-2, and SeO2 2- carry a 2- charge, indicating the addition of two extra electrons.
It is important to note that the molecular geometry, bond angles, and polarity of these selenium oxides can vary. The molecular geometry and bond angles depend on the number of bonded atoms and lone pairs around the central selenium atom. The polarity of the molecules is determined by the electronegativity difference between selenium and oxygen.
To understand the detailed molecular geometry, bond angles, and polarity of SeO2 and other selenium oxides, further analysis and calculations are required. These factors play a crucial role in determining the chemical properties and behavior of these compounds.
Frequently Asked Questions about SeO2 Lewis Structure
SeO2, also known as selenium dioxide, is a chemical compound composed of selenium and oxygen atoms. Understanding its Lewis structure is essential in comprehending its chemical properties and behavior. Here are some frequently asked questions about the SeO2 Lewis structure:
Valence electrons in SeO2
To determine the number of valence electrons in SeO2, we need to consider the valence electrons of each atom. Selenium (Se) belongs to Group 16 of the periodic table and has 6 valence electrons, while oxygen (O) belongs to Group 16 as well and has 6 valence electrons. Therefore, SeO2 has a total of 20 valence electrons.
Lewis dot structure of SeO2
The Lewis dot structure of SeO2 illustrates the arrangement of valence electrons around the atoms. In SeO2, the selenium atom is the central atom, surrounded by two oxygen atoms. Each oxygen atom forms a double bond with selenium, resulting in a total of two double bonds. The remaining two valence electrons on selenium are represented as lone pairs.
Sulfur Dioxide Lewis structure
It’s important to note that SeO2 is different from sulfur dioxide (SO2). While both compounds contain oxygen and sulfur atoms, they have distinct Lewis structures and molecular geometries. The Lewis structure of SO2 consists of a sulfur atom bonded to two oxygen atoms, with one double bond and one lone pair on the sulfur atom.
SeO2 molecular geometry
The molecular geometry of SeO2 is bent or V-shaped. The presence of two bonding pairs and two lone pairs of electrons around the central selenium atom gives rise to this shape. The bond angles in SeO2 are approximately 119 degrees.
Resonance structures of SeO2
SeO2 does not exhibit resonance structures. Resonance occurs when multiple Lewis structures can be drawn for a molecule by moving electrons. In the case of SeO2, the arrangement of atoms and electrons is fixed, and resonance is not observed.
Polar or nonpolar SeO2
SeO2 is a polar molecule. The bent molecular geometry and the presence of lone pairs on the central selenium atom result in an uneven distribution of charge. The oxygen atoms are more electronegative than selenium, causing a partial negative charge on the oxygen atoms and a partial positive charge on the selenium atom.
Octet rule in SeO2
The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight valence electrons. In the case of SeO2, the selenium atom shares electrons with the oxygen atoms to complete its octet. Each oxygen atom also completes its octet by sharing electrons with selenium.
Chemical bonding in SeO2
SeO2 exhibits covalent bonding. Covalent bonds are formed when atoms share electrons to achieve a stable electron configuration. In SeO2, the selenium atom shares electrons with the oxygen atoms, resulting in the formation of double bonds.
Electron pair geometry in SeO2
The electron pair geometry of SeO2 is trigonal planar. This geometry is determined by considering both the bonding and lone pairs of electrons around the central selenium atom.
SeO2 molecular shape
The molecular shape of SeO2 is bent or V-shaped. This shape is a result of the repulsion between the bonding and lone pairs of electrons around the central selenium atom.
Lone pairs in SeO2 structure
SeO2 has two lone pairs of electrons on the central selenium atom. These lone pairs contribute to the overall molecular shape and polarity of the molecule.
SeO2 oxidation state
In SeO2, the oxidation state of selenium is +4. Oxygen has an oxidation state of -2, and since there are two oxygen atoms, the total oxidation state contributed by oxygen is -4. The sum of the oxidation states in a neutral molecule is zero, so the oxidation state of selenium must be +4 to balance the charges.
Hybridization of SeO2
The hybridization of the central selenium atom in SeO2 is sp3. This hybridization allows the selenium atom to form four electron pairs, including two bonding pairs and two lone pairs.
For more detailed information on SeO2 Lewis structure and related topics, you can visit this link. It provides a comprehensive explanation of the molecular structure and properties of SeO2.
Remember, understanding the Lewis structure of SeO2 is crucial in understanding its chemical behavior and interactions.
References
Valence electrons in SEO2
In the molecule SEO2, selenium (Se) is the central atom surrounded by two oxygen (O) atoms. To determine the number of valence electrons in SEO2, we need to consider the electron configuration of selenium. Selenium is in Group 16 of the periodic table, so it has six valence electrons. Each oxygen atom contributes six valence electrons as well, giving SEO2 a total of 18 valence electrons.
Lewis dot structure of SEO2
The Lewis dot structure of SEO2 shows the arrangement of atoms and valence electrons in the molecule. To draw the Lewis dot structure, we start by placing the selenium atom in the center and connecting it to the two oxygen atoms with single bonds. Each oxygen atom is then surrounded by two lone pairs of electrons. The Lewis dot structure of SEO2 can be represented as Se-O-O, with the lone pairs depicted as dots around the oxygen atoms.
Sulfur Dioxide Lewis structure
Sulfur dioxide (SO2) is a similar molecule to SEO2, but with sulfur (S) as the central atom instead of selenium. The Lewis dot structure of sulfur dioxide is represented as S=O, with a double bond between sulfur and one oxygen atom, and a lone pair of electrons on the sulfur atom. This difference in structure affects the properties and behavior of the two molecules.
SEO2 molecular geometry
The molecular geometry of SEO2 is bent or V-shaped. This shape arises from the arrangement of the atoms and lone pairs around the central selenium atom. The two oxygen atoms are positioned in a bent shape, with the selenium atom at the center. The presence of lone pairs on the oxygen atoms causes a distortion in the molecular geometry, resulting in the bent shape.
Bond angles in SEO2
The bond angles in SEO2 are approximately 119 degrees. This angle is slightly less than the ideal angle of 120 degrees due to the repulsion between the lone pairs of electrons on the oxygen atoms. The presence of lone pairs causes a compression of the bond angles, resulting in a slightly smaller angle than expected.
Resonance structures of SEO2
Resonance structures are different representations of a molecule that can be drawn by moving electrons within the molecule. In the case of SEO2, there are no resonance structures due to the absence of multiple bonds or delocalized electrons. The Lewis dot structure accurately represents the arrangement of atoms and electrons in SEO2.
Polar or nonpolar SEO2
SEO2 is a polar molecule. The polarity arises from the bent molecular geometry and the unequal distribution of electron density. The oxygen atoms are more electronegative than the selenium atom, causing a partial negative charge on the oxygen atoms and a partial positive charge on the selenium atom. This uneven distribution of charges results in a polar molecule.
Octet rule in SEO2
The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight valence electrons. In the case of SEO2, the selenium atom shares electrons with the oxygen atoms to complete its octet. Each oxygen atom also completes its octet by sharing electrons with the selenium atom. This sharing of electrons satisfies the octet rule for all atoms in SEO2.
Chemical bonding in SEO2
The chemical bonding in SEO2 is covalent. Covalent bonds are formed when atoms share electrons to achieve a stable electron configuration. In SEO2, the selenium atom shares electrons with the oxygen atoms, resulting in the formation of two covalent bonds. The sharing of electrons allows each atom to complete its octet and achieve a more stable configuration.
Electron pair geometry in SEO2
The electron pair geometry of SEO2 is trigonal planar. This geometry describes the arrangement of all electron pairs, including both bonding and lone pairs, around the central selenium atom. In SEO2, the two oxygen atoms and the two lone pairs of electrons on the oxygen atoms are arranged in a trigonal planar shape around the selenium atom.
SEO2 molecular shape
The molecular shape of SEO2 is bent or V-shaped. This shape is determined by considering only the positions of the atoms, excluding the lone pairs of electrons. The presence of lone pairs on the oxygen atoms causes a distortion in the molecular shape, resulting in the bent or V-shaped structure.
Lone pairs in SEO2 structure
In the structure of SEO2, there are two lone pairs of electrons on each oxygen atom. These lone pairs are not involved in bonding and are localized on the oxygen atoms. The presence of lone pairs affects the molecular geometry and shape of SEO2, causing it to be bent or V-shaped.
SEO2 oxidation state
The oxidation state of selenium in SEO2 is +4. The oxidation state represents the charge that an atom would have if all the shared electrons were assigned to the more electronegative atom. In SEO2, each oxygen atom is more electronegative than selenium, so the shared electrons are assigned to the oxygen atoms. This results in a +4 oxidation state for selenium.
Hybridization of SEO2
The hybridization of selenium in SEO2 is sp3. Hybridization refers to the mixing of atomic orbitals to form new hybrid orbitals that are used for bonding. In SEO2, the s and p orbitals of selenium hybridize to form four sp3 hybrid orbitals. These hybrid orbitals are used to form sigma bonds with the oxygen atoms.
SEO2 Lewis structure explanation
The Lewis structure of SEO2 represents the arrangement of atoms and valence electrons in the molecule. It shows the sharing of electrons between the selenium and oxygen atoms, as well as the presence of lone pairs on the oxygen atoms. The Lewis structure provides a visual representation of the bonding and electron distribution in SEO2.
For more information on the Lewis dot structures and molecular geometries of molecules, you can visit this link which provides a comprehensive account of advanced topics in chemistry, including Lewis dot structures and molecular geometries.
Frequently Asked Questions
1. What is the Lewis dot structure of SeO2?
The Lewis dot structure of SeO2, or selenium dioxide, consists of a selenium atom at the center bonded to two oxygen atoms. The selenium atom has two lone pairs of electrons, and each oxygen atom is double-bonded to the selenium atom.
2. How can we determine the hybridization of SeO2 from its Lewis structure?
The hybridization of SeO2 can be determined from its Lewis structure by counting the number of sigma bonds and lone pairs around the central atom. In SeO2, the selenium atom forms two sigma bonds with the oxygen atoms and has two lone pairs, which corresponds to sp3 hybridization.
3. What is the molecular geometry of SeO2?
The molecular geometry of SeO2, based on its Lewis structure, is bent or V-shaped. This is due to the presence of two bonding pairs and two lone pairs of electrons around the central selenium atom.
4. Is SeO2 polar or nonpolar?
SeO2 is a polar molecule. This is because the molecule has a bent shape, leading to an uneven distribution of electron density and a net dipole moment.
5. What are the bond angles in SeO2?
The bond angles in SeO2 are less than 109.5 degrees due to the presence of two lone pairs on the selenium atom, which repel the bonded electron pairs and decrease the bond angle.
6. Does SeO2 have a resonance structure?
Yes, SeO2 does have resonance structures. The double bonds between the selenium and oxygen atoms can be placed in different locations, leading to different valid Lewis structures for the molecule.
7. How does the octet rule apply to SeO2?
In the case of SeO2, the central selenium atom follows the expanded octet rule, where it can have more than eight electrons in its valence shell. This is possible because selenium is in the third period of the periodic table and can use d orbitals for bonding.
8. What is the role of valence electrons in the SeO2 Lewis structure?
The valence electrons in SeO2 are involved in forming bonds with the oxygen atoms and also exist as lone pairs on the selenium atom. They are represented as dots in the Lewis structure.
9. What is the electron pair geometry in SeO2?
The electron pair geometry in SeO2 is tetrahedral, which includes both the bonding pairs and lone pairs of electrons around the central atom.
10. Can you explain the SeO2 Lewis structure?
In the SeO2 Lewis structure, the central selenium atom is bonded to two oxygen atoms through double bonds. There are also two lone pairs of electrons on the selenium atom. The molecule follows the expanded octet rule and has a bent molecular geometry due to the presence of the lone pairs.
Also Read: