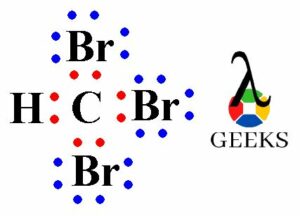
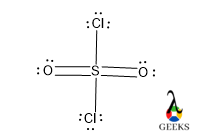
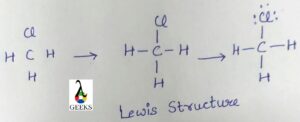
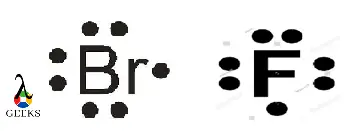The Lewis structure of ClO3, also known as chlorine trioxide, is a diagram that represents the arrangement of atoms and electrons in the molecule. In this structure, chlorine (Cl) is bonded to three oxygen (O) atoms. The central chlorine atom is surrounded by three oxygen atoms, each forming a single bond. The remaining electrons are represented as lone pairs on the oxygen atoms. The Lewis structure helps us understand the bonding and electron distribution in the molecule.
Key Takeaways
The table below provides a concise overview of the key information regarding the Lewis structure of ClO3:
| Atom | Number of Valence Electrons |
|---|---|
| Chlorine (Cl) | 7 |
| Oxygen (O) | 6 |
Please note that the Lewis structure is a simplified representation and does not account for the three-dimensional shape of the molecule.
Understanding the Lewis Structure
The Lewis structure is a visual representation of the arrangement of atoms and electrons in a molecule. It helps us understand the bonding and electron distribution in a compound. In this section, we will explore the Lewis dot structure of the chlorate anion (ClO3–) and discuss its valence electrons, lone pairs, and formal charge.
Lewis Dot Structure of Chlorate anion, ClO3–
To determine the Lewis dot structure of the chlorate anion (ClO3–), we need to know the number of valence electrons for each atom. Chlorine (Cl) belongs to Group 7A and has 7 valence electrons, while oxygen (O) belongs to Group 6A and has 6 valence electrons. Since there are three oxygen atoms in the chlorate anion, we have a total of 21 valence electrons (7 from chlorine + 6 from each oxygen).
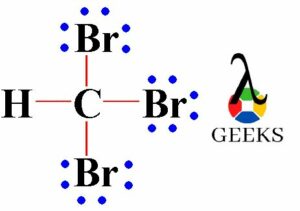
To represent the Lewis dot structure, we start by placing the central atom, chlorine (Cl), in the center. We then arrange the oxygen atoms (O) around it, making sure to distribute the valence electrons evenly. Each oxygen atom is bonded to the chlorine atom by a single bond, and the remaining valence electrons are placed as lone pairs on the oxygen atoms.
The Lewis dot structure of the chlorate anion (ClO3–) can be represented as follows:
O
||
O--Cl--O
||
O
Valence electrons in Chlorate anion, ClO3–

The chlorate anion (ClO3–) consists of one chlorine atom and three oxygen atoms. Chlorine has 7 valence electrons, while each oxygen atom has 6 valence electrons. Therefore, the total number of valence electrons in the chlorate anion is 21 (7 from chlorine + 6 from each oxygen).
Lone pair of electrons in Chlorate anion, ClO3–
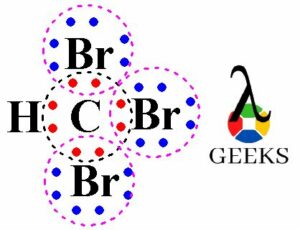
In the Lewis dot structure of the chlorate anion (ClO3–), each oxygen atom has two lone pairs of electrons. These lone pairs are represented by pairs of dots around the oxygen atoms. Lone pairs are important because they affect the molecular geometry and can influence the reactivity of a molecule.
Formal charge in Chlorate anion, ClO3–

To determine the formal charge in the chlorate anion (ClO3–), we compare the number of valence electrons assigned to an atom in the Lewis structure with its usual number of valence electrons. The formal charge helps us understand the distribution of electrons within a molecule.
In the case of the chlorate anion (ClO3–), the chlorine atom has a formal charge of 0, while each oxygen atom has a formal charge of -1. The sum of the formal charges in the molecule should equal the overall charge of the anion, which is -1.
By understanding the Lewis dot structure, valence electrons, lone pairs, and formal charge of the chlorate anion (ClO3–), we can gain insights into its chemical bonding, molecular geometry, and electron distribution. This knowledge is essential in the field of chemistry education and the study of molecular models, atomic orbitals, hybridization, and molecular polarity.
The Octet Rule and Resonance
Octet Rule in Chlorate anion, ClO3–


The Octet Rule is a fundamental principle in chemistry that states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with eight valence electrons. This rule helps us understand the formation of chemical bonds and the stability of molecules.
Let’s take a closer look at the Chlorate anion, ClO3–, to understand how the Octet Rule applies. Chlorate ion is composed of one chlorine atom (Cl) and three oxygen atoms (O). Chlorine has 7 valence electrons, while oxygen has 6 valence electrons. To achieve an octet, chlorine needs one more electron, while each oxygen atom needs two more electrons.
To satisfy the Octet Rule, the chlorine atom in the Chlorate anion can form three covalent bonds with three oxygen atoms. Each oxygen atom shares one of its electrons with the chlorine atom, resulting in a total of three bonding pairs. This arrangement allows the chlorine atom to achieve an octet of electrons, while each oxygen atom also achieves an octet.
Resonance in Chlorate anion, ClO3–
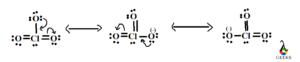
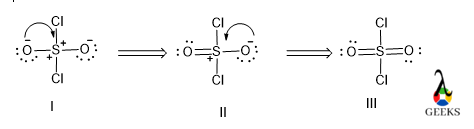
Resonance is a concept used to describe the delocalization of electrons in molecules or ions. In the case of the Chlorate anion, ClO3–, resonance occurs due to the presence of multiple equivalent Lewis structures that can be drawn for the molecule.
When we draw the Lewis dot structure for the Chlorate anion, we find that we can distribute the three oxygen atoms around the central chlorine atom in different ways. Each oxygen atom can take turns being double-bonded to the chlorine atom, resulting in three possible resonance structures.
The resonance structures of the Chlorate anion show that the bonding electrons are delocalized, meaning they are not fixed between specific atoms but rather spread out over the molecule. This delocalization contributes to the stability of the Chlorate anion.
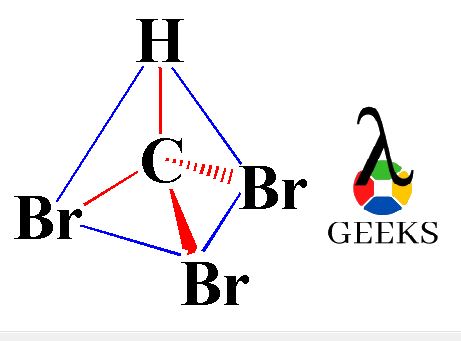
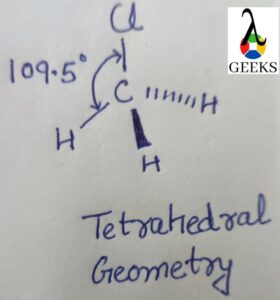
In terms of molecular geometry, the Chlorate anion has a trigonal pyramidal electron pair geometry and a tetrahedral molecular geometry. The VSEPR theory helps us understand the arrangement of electron pairs around the central chlorine atom. There are three bonding pairs and one non-bonding pair of electrons, also known as a lone pair.
The concept of resonance and the Octet Rule are crucial in understanding chemical bonding and molecular models. By considering the electron distribution and hybridization of atomic orbitals, we can determine the molecular polarity and predict the behavior of molecules in various chemical reactions.
Characteristics of Chlorate Anion
The Chlorate ion (ClO3-) is a polyatomic ion that consists of one chlorine atom bonded to three oxygen atoms. It exhibits several interesting characteristics, including its shape and angle, hybridization, and solubility.
Shape and angle of ClO3–
In terms of molecular geometry, the Chlorate ion (ClO3-) has a trigonal pyramidal shape. This means that the three oxygen atoms are arranged in a triangular shape around the central chlorine atom. The angle between the chlorine-oxygen bonds is approximately 109.5 degrees, which is consistent with the expected angle for a trigonal pyramidal structure.
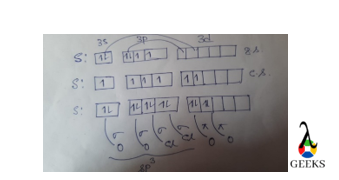
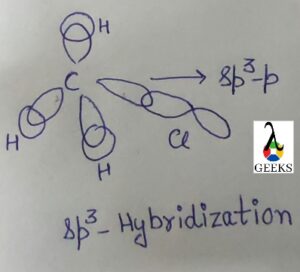
Hybridization in Chlorate anion, ClO3–

To understand the hybridization in the Chlorate ion (ClO3-), we need to consider the Lewis dot structure and the valence electrons of the atoms involved. The Lewis dot structure of ClO3- shows that the central chlorine atom is surrounded by three oxygen atoms, each contributing one electron to form a single bond with chlorine. Additionally, the chlorine atom has one lone pair of electrons.
According to the VSEPR theory, the electron pair geometry of ClO3- is tetrahedral, while the molecular geometry is trigonal pyramidal. This suggests that the chlorine atom undergoes sp3 hybridization, where one 3s orbital and three 3p orbitals hybridize to form four sp3 hybrid orbitals. These hybrid orbitals then overlap with the oxygen’s p orbitals to form the chlorine-oxygen sigma bonds.
Solubility of Chlorate anion, ClO3–
The solubility of the Chlorate ion (ClO3-) depends on various factors, including the nature of the solvent and the presence of other ions. Generally, Chlorate salts are highly soluble in water due to the strong electrostatic interactions between the ions and the polar water molecules. This solubility allows for the easy dissociation of the Chlorate ion into its constituent ions in aqueous solutions.
It is important to note that the solubility of Chlorate salts can vary depending on the specific cation present. For example, alkali metal chlorates (such as sodium chlorate and potassium chlorate) are highly soluble in water, while some other metal chlorates may have lower solubilities.
Properties of Chlorate Anion
The Chlorate ion (ClO3–) is an important chemical species in chemistry education. It exhibits several interesting properties that are worth exploring. Let’s delve into some of these properties and understand the nature of the Chlorate anion.
Is Chlorate anion, ClO3– Polar or not?
To determine whether the Chlorate anion is polar or not, we need to consider its molecular geometry and the distribution of its electrons. The Chlorate ion has a trigonal pyramidal molecular geometry due to the presence of three oxygen atoms bonded to a central chlorine atom. This arrangement results in a net dipole moment, making the Chlorate anion polar.
Is Chlorate anion, ClO3– Ionic or not?
The Chlorate anion is not purely ionic. It is formed through covalent bonding between the central chlorine atom and the surrounding oxygen atoms. However, the electronegativity difference between chlorine and oxygen is significant enough to create a partial ionic character in the Chlorate ion.
Is Chlorate anion, ClO3– Acidic or not?
The Chlorate anion is not acidic in nature. It does not readily donate protons (H+) to a solution. Instead, it can act as a base by accepting protons. The presence of lone pairs on the oxygen atoms allows the Chlorate ion to form hydrogen bonds with proton-donating species.
Is Chlorate anion, ClO3– Tetrahedral or Linear?
The Chlorate anion (ClO3–) has a trigonal pyramidal molecular geometry, which is a three-dimensional arrangement. It is not linear. The central chlorine atom is bonded to three oxygen atoms, resulting in a tetrahedral electron pair geometry. The presence of a lone pair on one of the oxygen atoms gives it a trigonal pyramidal shape.
Detailed Analysis of ClO3- Lewis Structure
The Lewis dot structure of the Chlorate ion (ClO3-) is a useful tool in understanding its molecular geometry and chemical bonding. By examining the arrangement of valence electrons and the resonance structures, we can gain insights into the electron distribution and overall structure of ClO3-.
ClO3- Lewis Structure Molecular Geometry
To determine the molecular geometry of ClO3-, we first need to understand its Lewis dot structure. The Chlorate ion consists of one central chlorine atom (Cl) bonded to three oxygen atoms (O). The Lewis dot structure represents the valence electrons of each atom as dots or lines.
In the case of ClO3-, the Lewis dot structure shows that the central chlorine atom is surrounded by three oxygen atoms. Each oxygen atom is connected to the chlorine atom by a single bond, and there are also three lone pairs of electrons on each oxygen atom. This arrangement gives ClO3- a trigonal pyramidal molecular geometry.
ClO3- Lewis Structure Valence Electrons
Valence electrons are the electrons in the outermost shell of an atom that participate in chemical bonding. In the case of ClO3-, the chlorine atom has 7 valence electrons, and each oxygen atom has 6 valence electrons. Therefore, the total number of valence electrons in ClO3- can be calculated as follows:
1 chlorine atom (7 valence electrons) + 3 oxygen atoms (6 valence electrons each) = 25 valence electrons
ClO3- Lewis Structure Lone Pairs
Lone pairs of electrons are non-bonding electrons that are not involved in chemical bonding. In the Lewis dot structure of ClO3-, each oxygen atom has three lone pairs of electrons. These lone pairs contribute to the overall electron distribution and influence the molecular geometry of ClO3-.
ClO3- Lewis Structure that Obeys Octet Rule
The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with eight valence electrons. In the case of ClO3-, the central chlorine atom has seven valence electrons and forms single bonds with three oxygen atoms, resulting in a total of ten valence electrons around the chlorine atom. This exceeds the octet rule.
To accommodate the extra electrons, ClO3- exhibits resonance structures. Resonance occurs when multiple Lewis structures can be drawn for a molecule by moving electrons around. In the case of ClO3-, the double bonds can be alternated between the chlorine atom and the oxygen atoms, resulting in three resonance structures.
ClO3- Lewis Structure Resonance
Resonance structures are different representations of a molecule that can be interconverted by moving electrons. In the case of ClO3-, the three resonance structures show the distribution of double bonds between the chlorine atom and the oxygen atoms. This resonance delocalizes the electrons and contributes to the stability of the molecule.
By considering the molecular geometry, valence electrons, lone pairs, adherence to the octet rule, and resonance structures, we can gain a comprehensive understanding of the ClO3- Lewis structure. This analysis provides valuable insights into the chemical bonding and electron distribution within the Chlorate ion.
For further exploration of ClO3- and other molecular structures, the concepts of VSEPR theory, bonding electrons, non-bonding electrons, atomic orbitals, hybridization, molecular polarity, and other aspects of chemistry education can be studied. These concepts help in constructing molecular models and understanding the behavior of different compounds.
Additional Information on ClO3- Lewis Structure
The Chlorate ion (ClO3-) is a polyatomic ion that consists of one chlorine atom bonded to three oxygen atoms. Understanding its Lewis dot structure is essential in comprehending its chemical properties and behavior.
Does ClO3- have a dipole moment?
Yes, ClO3- does have a dipole moment. A dipole moment occurs when there is an uneven distribution of electron density within a molecule. In the case of ClO3-, the chlorine atom pulls the electrons towards itself, creating a partial negative charge, while the oxygen atoms have a partial positive charge. This unequal distribution of charges results in a dipole moment.
Is ClO3- planar?
No, ClO3- is not planar. The Lewis structure of ClO3- reveals that the central chlorine atom is bonded to three oxygen atoms. The arrangement of these atoms gives rise to a trigonal pyramidal shape, where the chlorine atom occupies the apex of the pyramid, and the three oxygen atoms form the base.
How many lone pairs does ClO3- have?
ClO3- has one lone pair of electrons. In the Lewis structure, the chlorine atom has three bonding pairs, each shared with an oxygen atom, and one non-bonding pair of electrons. The presence of this lone pair contributes to the overall molecular geometry and affects the polarity of the molecule.
ClO3- Lewis structure shape
The Lewis structure of ClO3- suggests a trigonal pyramidal shape. This shape is determined by the arrangement of the bonding and non-bonding electron pairs around the central chlorine atom. The three oxygen atoms are positioned in a triangular base, while the lone pair of electrons occupies the apex of the pyramid.
ClO3- best Lewis structure
The best Lewis structure for ClO3- involves the concept of resonance structures. Resonance occurs when there are multiple ways to arrange the electrons in a molecule without violating the octet rule. In the case of ClO3-, the three oxygen atoms can each form a double bond with the chlorine atom in different arrangements. These resonance structures contribute to the stability of the molecule and its overall behavior.
References and Further Reading
In order to understand the concept of Chlorate ion (ClO3-), it is important to have a solid grasp of various topics such as Lewis dot structure, valence electrons, resonance structures, molecular geometry, electron pair geometry, VSEPR theory, bonding electrons, non-bonding electrons, octet rule, chemical bonding, molecular models, lone pairs, atomic orbitals, hybridization, molecular polarity, and electron distribution. These concepts are fundamental to the study of chemistry and play a crucial role in understanding the structure and properties of molecules.
To delve deeper into these topics, here are some references and further reading materials that can provide you with a comprehensive understanding:
-
“Chemistry: The Central Science” by Theodore L. Brown, H. Eugene LeMay, and Bruce E. Bursten – This textbook covers a wide range of topics in chemistry, including molecular structure and bonding. It provides clear explanations and examples to help you grasp the concepts.
-
“Chemical Bonding and Molecular Structure” by P. Bahadur – This book focuses specifically on chemical bonding and molecular structure. It covers topics such as Lewis structures, VSEPR theory, and hybridization in detail, making it a valuable resource for understanding molecular geometry.
-
“Inorganic Chemistry” by Gary L. Miessler, Paul J. Fischer, and Donald A. Tarr – This textbook provides a comprehensive overview of inorganic chemistry, including topics such as atomic structure, bonding, and molecular structure. It offers a thorough explanation of concepts related to electron distribution and molecular polarity.
-
“Chemistry Education Research and Practice” – This journal publishes research articles and studies related to chemistry education. It covers a wide range of topics, including teaching strategies, student learning, and curriculum development. It can provide valuable insights into effective ways of teaching and learning chemistry.
Additionally, online resources such as educational websites, interactive simulations, and video tutorials can also be helpful in gaining a deeper understanding of these concepts. Websites like Khan Academy, ChemGuide, and ChemSpider offer a wealth of information and resources for studying chemistry.
By exploring these references and further reading materials, you can enhance your knowledge and understanding of Chlorate ion (ClO3-) and related concepts in chemistry. Happy learning!
Frequently Asked Questions
What is the Lewis structure for ClO3-?
The Lewis structure for ClO3- consists of a central Chlorine (Cl) atom surrounded by three Oxygen (O) atoms. The Chlorine atom forms single bonds with each Oxygen atom. The remaining valence electrons are distributed as lone pairs on the Oxygen atoms to fulfill the octet rule.
What is the molecular geometry of ClO3- based on its Lewis structure?
The molecular geometry of ClO3- is trigonal pyramidal. This is determined using the VSEPR (Valence Shell Electron Pair Repulsion) theory, which states that electron pairs around a central atom will arrange themselves in such a way that they minimize repulsion.
How many valence electrons are there in the ClO3- Lewis structure?
In the ClO3- Lewis structure, there are a total of 24 valence electrons. Chlorine contributes 7 electrons, and each of the three Oxygen atoms contributes 6 electrons.
How many lone pairs are there in the ClO3- Lewis structure?
In the ClO3- Lewis structure, there are a total of 8 lone pairs. Each Oxygen atom has two lone pairs, and the central Chlorine atom has one lone pair.
Does the ClO3- Lewis structure obey the octet rule?
Yes, the ClO3- Lewis structure obeys the octet rule. Each Oxygen atom is surrounded by 8 electrons (2 from the bond with Chlorine and 6 from lone pairs), and the central Chlorine atom is also surrounded by 8 electrons (3 from bonds with Oxygen and 2 from a lone pair).
Does ClO3- have a dipole moment?
Yes, ClO3- has a dipole moment due to its trigonal pyramidal geometry and the difference in electronegativity between Chlorine and Oxygen. This makes ClO3- a polar molecule.
What is the formal charge on ClO3- based on its Lewis structure?
The formal charge on ClO3- is -1. This is calculated by subtracting the number of valence electrons in the isolated atom from the number of valence electrons assigned to the atom in the Lewis structure.
Does ClO3- have resonance structures?
Yes, ClO3- has resonance structures. These are different ways of arranging the electrons in the molecule that still satisfy the octet rule. In the case of ClO3-, the resonance structures involve different Oxygen atoms forming double bonds with the central Chlorine atom.
Is ClO3- planar based on its Lewis structure?
No, ClO3- is not planar. Its trigonal pyramidal molecular geometry, as determined by the VSEPR theory, involves a three-dimensional arrangement of atoms.
What is the bond angle in the ClO3+ Lewis structure?
The bond angle in the ClO3+ Lewis structure is approximately 109.5 degrees, which is characteristic of a tetrahedral electron pair geometry.
Also Read: