Ammonia (NH3) is a compound that is commonly encountered in various everyday situations, such as cleaning products, fertilizers, and even in our own bodies. It is a colorless gas with a pungent odor and is known for its ability to dissolve in water, forming a weakly alkaline solution. In the world of chemistry, the question often arises: Is NH3 an acid or a base? The answer lies in understanding the properties of acids and bases and how NH3 behaves in different chemical reactions. In this article, we will explore the nature of NH3 and delve into its acid-base properties, shedding light on its behavior and significance in various contexts. So, let’s dive in and uncover the secrets of NH3 as an acid or base.
Key Takeaways
- NH3 is a weak base that can accept a proton (H+) to form NH4+.
- NH3 can also act as a Lewis base by donating a lone pair of electrons.
- In water, NH3 can undergo partial ionization to form NH4+ and OH- ions.
- NH3 is commonly used in household cleaning products and as a refrigerant.
- Understanding the properties of NH3 as an acid or base is important in various chemical reactions and applications.
Is Ammonia Acidic, Neutral, or Basic?
Ammonia, also known as NH3, is a compound that is commonly used in various industries and household products. It is important to understand the nature of ammonia and whether it is acidic, neutral, or basic. In this section, we will explore the basic nature of ammonia, its pH value, and its ability to act as an amphoteric compound.
Explanation of Ammonia’s Basic Nature
Ammonia is considered a basic compound due to its ability to accept protons (H+) and form ammonium ions (NH4+). In a chemical reaction, ammonia acts as a Lewis base, which means it can donate a pair of electrons to form a bond with a Lewis acid. This property allows ammonia to neutralize acids and form salts.
When ammonia dissolves in water, it reacts with water molecules to form ammonium ions and hydroxide ions (OH-). The reaction can be represented as follows:
NH3 + H2O ⇌ NH4+ + OH-
The formation of hydroxide ions in this reaction contributes to the basic nature of ammonia. The presence of hydroxide ions in a solution increases its pH value, indicating alkalinity.
pH Value of Ammonia Indicating its Basicity
The pH scale is a measure of the acidity or alkalinity of a solution. It ranges from 0 to 14, with 7 being considered neutral. Solutions with a pH value below 7 are acidic, while solutions with a pH value above 7 are basic or alkaline.
Ammonia has a pH value greater than 7, typically ranging from 11 to 12. This high pH value confirms its basic nature. When ammonia is dissolved in water, it increases the concentration of hydroxide ions, which in turn raises the pH of the solution.
Ammonia as an Amphoteric Compound
Ammonia exhibits amphoteric behavior, meaning it can act as both an acid and a base depending on the reaction it undergoes. As mentioned earlier, ammonia can accept protons to form ammonium ions, which makes it a base. On the other hand, ammonia can also donate a pair of electrons to form a bond with a Lewis acid, making it an acid.
For example, when ammonia reacts with a strong acid, such as hydrochloric acid (HCl), it acts as a base and accepts a proton from the acid to form the ammonium ion (NH4+):
NH3 + HCl → NH4+ + Cl-
Conversely, when ammonia reacts with a strong base, such as sodium hydroxide (NaOH), it acts as an acid and donates a pair of electrons to form a bond with the hydroxide ion (OH-):
NH3 + OH- → NH2- + H2O
This ability to act as both an acid and a base makes ammonia a versatile compound in various chemical reactions.
In conclusion, ammonia is a basic compound due to its ability to accept protons and form ammonium ions. Its pH value is greater than 7, indicating its alkaline nature. Additionally, ammonia exhibits amphoteric behavior, allowing it to act as both an acid and a base in different chemical reactions. Understanding the basic nature of ammonia is essential in various applications, from industrial processes to household cleaning products.
Is NH3 a Good Base?
Ammonia (NH3) is commonly known as a weak base. In this section, we will discuss the strength of ammonia as a base and compare it with strong bases like potassium hydroxide and sodium hydroxide.
Discussion on the Strength of Ammonia as a Base
When we talk about the strength of a base, we are referring to its ability to accept protons (H+) or donate electron pairs. In the case of ammonia, it acts as a base by accepting a proton from a water molecule to form the ammonium ion (NH4+). This reaction can be represented as follows:
NH3 + H2O ⇌ NH4+ + OH-
Ammonia is classified as a weak base because it does not completely dissociate in water and only a small fraction of ammonia molecules react with water to form the ammonium ion. This means that ammonia is not as effective at accepting protons compared to strong bases.
Comparison with Strong Bases like Potassium Hydroxide and Sodium Hydroxide
Strong bases, such as potassium hydroxide (KOH) and sodium hydroxide (NaOH), have a higher affinity for protons and are more effective at accepting them. These bases completely dissociate in water, producing hydroxide ions (OH-) that can readily accept protons.
For example, when potassium hydroxide is dissolved in water, it dissociates completely into potassium ions (K+) and hydroxide ions (OH-):
KOH → K+ + OH-
Similarly, sodium hydroxide dissociates into sodium ions (Na+) and hydroxide ions (OH-):
NaOH → Na+ + OH-
Due to their complete dissociation, strong bases like potassium hydroxide and sodium hydroxide are much more effective at accepting protons compared to ammonia. This makes them stronger bases in terms of their ability to neutralize acids and increase the pH of a solution.
In summary, ammonia is considered a weak base because it only partially dissociates in water and has a lower affinity for protons compared to strong bases like potassium hydroxide and sodium hydroxide.
NH3 Acid or Base: Strong or Weak?
Explanation of Ammonia as a Weak Base
Ammonia, with the chemical formula NH3, is a compound that is commonly encountered in everyday life. It is a colorless gas with a pungent odor and is often used in cleaning products, fertilizers, and even as a refrigerant. But when it comes to its chemical properties, is NH3 an acid or a base?
In the context of acid-base chemistry, ammonia is considered a weak base. A base is a substance that can accept a proton (H+) from an acid, and in the case of ammonia, it can accept a proton to form the ammonium ion (NH4+). This ability to accept a proton is what classifies ammonia as a base.
Definition of Strong and Weak Bases
To understand why ammonia is classified as a weak base, it is important to have a clear understanding of what constitutes a strong or weak base. In general, a base can be classified as either strong or weak based on its ability to accept protons.
A strong base is one that readily accepts protons and completely dissociates in water, producing hydroxide ions (OH-) as the conjugate base. Examples of strong bases include sodium hydroxide (NaOH) and potassium hydroxide (KOH). These bases have a high affinity for protons and easily release hydroxide ions in solution.
On the other hand, a weak base, like ammonia, has a lower affinity for protons and does not completely dissociate in water. Instead, it only partially accepts protons, forming the ammonium ion (NH4+) as the conjugate acid. This partial dissociation is what characterizes a weak base.
Examples of Weak Bases like Ammonium Hydroxide
Ammonium hydroxide, also known as ammonia water, is a common example of a weak base. It is a solution of ammonia dissolved in water and is often used as a cleaning agent or in various industrial processes.
When ammonium hydroxide is dissolved in water, it undergoes a partial dissociation, releasing some ammonium ions (NH4+) and hydroxide ions (OH-). The equilibrium between ammonia and ammonium ions is established, resulting in a weakly basic solution.
The pH of a solution containing ammonium hydroxide will be slightly higher than 7, indicating its basic nature. However, since ammonia is a weak base, the concentration of hydroxide ions in the solution will be relatively low compared to a strong base like sodium hydroxide.
In summary, ammonia (NH3) is classified as a weak base due to its ability to accept protons and form the ammonium ion (NH4+). It is important to distinguish between strong and weak bases, as their properties and behavior in solution can vary significantly.
What is NH3 Acid or Base?
Ammonia (NH3) is a fascinating compound that exhibits both acidic and basic properties. In this section, we will explore how ammonia behaves as both an acid and a base, and the formation of NH4+ ions as a weak acid and NH2- ions as a conjugate base.
Explanation of Ammonia’s Behavior as Both an Acid and a Base
Ammonia is a compound composed of one nitrogen atom bonded to three hydrogen atoms. It is classified as a weak base due to its ability to accept protons (H+) from other substances. However, under certain conditions, ammonia can also act as a weak acid by donating protons.
When ammonia is dissolved in water, it can react with water molecules through a process called an acid-base reaction. In this reaction, ammonia acts as a base by accepting a proton from a water molecule, forming an ammonium ion (NH4+) and a hydroxide ion (OH-). The ammonium ion acts as a weak acid in this reaction.
Formation of NH4+ Ions as a Weak Acid
When ammonia accepts a proton from a water molecule, it forms the ammonium ion (NH4+). This reaction can be represented as follows:
NH3 + H2O → NH4+ + OH-
The formation of NH4+ ions as a weak acid is an example of a Bronsted-Lowry acid-base reaction. In this reaction, ammonia acts as a Bronsted-Lowry base, accepting a proton from water to form the ammonium ion.
Formation of NH2- Ions as a Conjugate Base
Ammonia can also act as a weak acid by donating a proton to a base. When ammonia donates a proton, it forms the amide ion (NH2-). This reaction can be represented as follows:
NH3 + Base → NH2- + Conjugate Acid
In this reaction, ammonia acts as a Bronsted-Lowry acid, donating a proton to a base to form the amide ion. The amide ion is the conjugate base of ammonia.
In summary, ammonia exhibits both acidic and basic properties. It can act as a weak acid by donating a proton and as a weak base by accepting a proton. The formation of NH4+ ions as a weak acid and NH2- ions as a conjugate base demonstrates the versatile nature of ammonia in acid-base reactions.
NH3 Acidic or Basic?
Ammonia (NH3) is a fascinating compound that is commonly encountered in various contexts, such as household cleaning products and fertilizers. One of the fundamental aspects of ammonia is its nature as either an acid or a base. In this section, we will explore the basic nature of ammonia and explain its ability to accept protons.
Confirmation of Ammonia’s Basic Nature
Ammonia is widely recognized as a base due to its behavior in acid-base reactions. When ammonia is dissolved in water, it readily accepts protons (H+) from water molecules, resulting in the formation of ammonium ions (NH4+). This reaction can be represented as follows:
NH3 + H2O ⇌ NH4+ + OH-
In this reaction, ammonia acts as a Bronsted-Lowry base by accepting a proton from water, which acts as an acid. The formation of hydroxide ions (OH-) further confirms the basic nature of ammonia.
Explanation of Ammonia’s Ability to Accept Protons
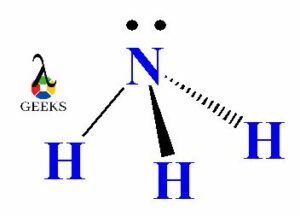
The ability of ammonia to accept protons stems from its molecular structure. Ammonia consists of one nitrogen atom bonded to three hydrogen atoms. The nitrogen atom has a lone pair of electrons, making it an ideal candidate for accepting a proton.
When ammonia accepts a proton, it forms a new compound called the ammonium ion (NH4+). The ammonium ion is a positively charged species, as it has gained an extra proton. This process is reversible, meaning that the ammonium ion can also donate a proton to a base, acting as a weak acid.
Ammonia’s ability to accept protons is not limited to aqueous solutions. It can also react with other acids, such as hydrochloric acid (HCl), to form ammonium chloride (NH4Cl). This reaction demonstrates ammonia’s versatility as a base in non-aqueous environments as well.
In addition to being a Bronsted-Lowry base, ammonia can also act as a Lewis base. A Lewis base is a species that donates a pair of electrons to form a covalent bond. In the case of ammonia, the lone pair of electrons on the nitrogen atom can form a bond with a Lewis acid, which is a species that accepts a pair of electrons. This ability to form covalent bonds further highlights ammonia’s basic nature.
In summary, ammonia is unequivocally a base due to its behavior in acid-base reactions and its ability to accept protons. Its molecular structure, which includes a lone pair of electrons on the nitrogen atom, allows it to readily accept protons and form ammonium ions. Whether in aqueous or non-aqueous environments, ammonia’s basic nature remains consistent, making it a crucial component in various chemical processes.
Why Does NH3 Act as a Base?
Ammonia (NH3) is a fascinating compound that exhibits basic properties in various chemical reactions. In this section, we will explore the reasons behind NH3 acting as a base and its behavior in different contexts.
Discussion on the Electron Pairs in Ammonia’s Structure
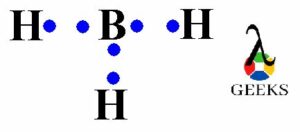
To understand why NH3 acts as a base, we need to delve into its molecular structure. Ammonia consists of one nitrogen atom bonded to three hydrogen atoms. The nitrogen atom has a lone pair of electrons, which is not involved in any bonding. This lone pair of electrons plays a crucial role in ammonia’s basic behavior.
Donation of Lone Electron Pairs to Other Compounds
One of the key characteristics of a base is its ability to donate a pair of electrons. In the case of ammonia, the lone pair of electrons on the nitrogen atom can be donated to other compounds. When ammonia encounters an acid, it can donate its lone pair of electrons to the acid, forming a new bond.
For example, when ammonia reacts with hydrochloric acid (HCl), the lone pair of electrons on the nitrogen atom is donated to the hydrogen ion (H+), resulting in the formation of the ammonium ion (NH4+). This reaction demonstrates ammonia’s basic nature, as it accepts a proton (H+) from the acid.
Consideration of Ammonia as a Lewis Base
In addition to its ability to donate a pair of electrons, ammonia can also be classified as a Lewis base. Lewis bases are compounds that can donate an electron pair to form a coordinate bond with a Lewis acid.
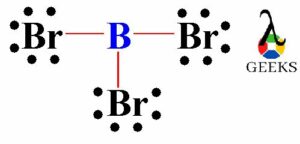
In the case of ammonia, the lone pair of electrons on the nitrogen atom can form a coordinate bond with a Lewis acid, such as boron trifluoride (BF3). The nitrogen atom donates its lone pair of electrons to the boron atom, resulting in the formation of a stable compound.
This behavior further highlights ammonia’s basic nature, as it can readily donate its lone pair of electrons to form new bonds with other compounds.
In summary, ammonia acts as a base due to the presence of a lone pair of electrons on its nitrogen atom. This lone pair can be donated to other compounds, allowing ammonia to exhibit basic behavior. Additionally, ammonia can also be considered a Lewis base, as it can form coordinate bonds with Lewis acids. Understanding the basic nature of ammonia is crucial in various chemical reactions and plays a significant role in many industrial processes.
NH3 Aq Acid or Base?
Ammonia, also known as NH3, is a compound that is commonly encountered in our daily lives. It is used in various applications, such as cleaning agents, fertilizers, and even as a refrigerant. But have you ever wondered whether ammonia behaves as an acid or a base when it is dissolved in water? Let’s explore the behavior of ammonia in water and understand its role in acid-base reactions.
Explanation of Ammonia’s Behavior in Water
When ammonia (NH3) is dissolved in water, it undergoes a fascinating transformation. The ammonia molecule can act as a base, meaning it can accept a proton (H+) from an acid. In this case, water acts as the acid, donating a proton to ammonia. This reaction can be represented as follows:
NH3 + H2O ⇌ NH4+ + OH-
Formation of Hydroxide (OH-) and Ammonium (NH4+) Ions
As we can see from the reaction above, when ammonia accepts a proton from water, it forms ammonium ions (NH4+) and hydroxide ions (OH-). The ammonium ion is a conjugate acid, while the hydroxide ion is a conjugate base. This reaction is reversible, meaning that the ammonium and hydroxide ions can also react to form ammonia and water again.
Equilibrium Between NH3, NH4+, H+, and OH- Ions
The equilibrium between ammonia, ammonium ions, hydrogen ions (protons), and hydroxide ions plays a crucial role in determining the acidity or basicity of a solution. In an aqueous solution of ammonia, there is a dynamic balance between these species. If the concentration of hydrogen ions (H+) is higher than the concentration of hydroxide ions (OH-), the solution is considered acidic. On the other hand, if the concentration of hydroxide ions is higher than the concentration of hydrogen ions, the solution is basic.
In the case of ammonia, the equilibrium lies more towards the left side of the reaction, meaning that ammonia is a weak base. This is because ammonia has a greater tendency to accept protons (H+) from water and form ammonium ions (NH4+) rather than donate protons. Therefore, ammonia is classified as a Bronsted-Lowry base, which is a substance that can accept a proton.
Summary
In summary, when ammonia (NH3) is dissolved in water, it acts as a base and accepts a proton from water, forming ammonium ions (NH4+) and hydroxide ions (OH-). The equilibrium between ammonia, ammonium ions, hydrogen ions, and hydroxide ions determines the acidity or basicity of the solution. Ammonia is classified as a weak base, as it has a greater tendency to accept protons rather than donate them. Understanding the behavior of ammonia in water is essential in comprehending acid-base reactions and the pH of solutions.
Now that we have explored the behavior of ammonia in water, let’s delve deeper into the concept of pH and its relationship with acid-base reactions.
Is NH3 Acidic, Basic, or Neutral?
Ammonia (NH3) is a compound that is commonly encountered in various contexts, such as household cleaning products, fertilizers, and even in our own bodies. Understanding its nature as an acid, base, or neutral substance is crucial in comprehending its behavior and reactions. In this section, we will explore the basic nature of ammonia and compare it with acidic and neutral substances.
Confirmation of Ammonia’s Basic Nature
Ammonia is classified as a base due to its ability to accept protons (H+) from acids. This property is a result of its molecular structure, which consists of a central nitrogen atom bonded to three hydrogen atoms. The lone pair of electrons on the nitrogen atom makes it an attractive site for accepting protons.
When ammonia reacts with water, it acts as a Bronsted-Lowry base, accepting a proton from water to form the ammonium ion (NH4+). This reaction can be represented as follows:
NH3 + H2O → NH4+ + OH-
The ammonium ion (NH4+) is the conjugate acid of ammonia, while the hydroxide ion (OH-) is the conjugate base. This reaction occurs in aqueous solutions and contributes to the alkaline nature of ammonia.
Comparison with Acidic and Neutral Substances
To better understand the basic nature of ammonia, let’s compare it with acidic and neutral substances.
Acidic Substances: Acids are substances that release protons (H+) when dissolved in water. They can be strong or weak, depending on the extent of proton release. Examples of common acids include hydrochloric acid (HCl) and sulfuric acid (H2SO4). Unlike ammonia, acids have a tendency to donate protons rather than accept them.
Neutral Substances: Neutral substances have an equal balance of acidic and basic properties. They neither donate nor accept protons in water. Water itself is a prime example of a neutral substance, as it can act as both an acid and a base. This property is known as amphotericity. Other neutral substances include salts and certain organic compounds.
In summary, ammonia is classified as a base due to its ability to accept protons from acids. Its basic nature is confirmed by its reaction with water, where it forms the ammonium ion and hydroxide ion. By comparing it with acidic and neutral substances, we can appreciate the unique characteristics of ammonia as a basic compound.
| Substance |
Nature |
| Ammonia (NH3) |
Basic |
| Hydrochloric acid (HCl) |
Acidic |
| Sulfuric acid (H2SO4) |
Acidic |
| Water (H2O) |
Neutral |
| Salts |
Neutral |
| Organic compounds |
Neutral |
Understanding the acid-base properties of ammonia is essential in various fields, including chemistry, biology, and environmental science. By grasping its basic nature, we can better comprehend its behavior and its role in different chemical reactions.
NH3 Acid or Base Name
Explanation of Ammonia’s Classification as a Base
When it comes to understanding the nature of ammonia (NH3), it is essential to delve into its classification as a base. In chemistry, substances are categorized as either acids or bases based on their behavior in chemical reactions. Ammonia falls into the category of bases due to its ability to accept protons (H+) from acids.
To comprehend why ammonia is considered a base, we need to explore the Bronsted-Lowry theory of acids and bases. According to this theory, an acid is a substance that donates protons, while a base is a substance that accepts protons. In the case of ammonia, it acts as a base by accepting a proton from an acid, forming a new compound.
Ammonia’s ability to accept protons is attributed to its lone pair of electrons on the nitrogen atom. This lone pair of electrons is available for bonding with a proton, resulting in the formation of the ammonium ion (NH4+). This reaction demonstrates ammonia’s base-like behavior.
Naming Conventions for Acids and Bases
In chemistry, naming conventions play a crucial role in identifying and categorizing different compounds. Acids and bases have specific naming rules that help distinguish them from other types of compounds.
Naming Acids
Acids are named based on the anion they contain. An anion is a negatively charged ion that is formed when an acid donates a proton. The naming of acids depends on whether the anion is a binary or oxyanion.
-
Binary Acids: Binary acids consist of hydrogen and a nonmetal. To name a binary acid, the prefix “hydro-” is added to the name of the nonmetal, followed by the suffix “-ic.” Finally, the word “acid” is appended. For example, HCl is hydrochloric acid, and HF is hydrofluoric acid.
-
Oxyacids: Oxyacids contain hydrogen, oxygen, and a nonmetal. The naming of oxyacids depends on the polyatomic ion present. If the polyatomic ion ends in “-ate,” the suffix “-ic” is added to the root name of the ion, followed by the word “acid.” For example, HNO3 is nitric acid. If the polyatomic ion ends in “-ite,” the suffix “-ous” is added to the root name of the ion, followed by the word “acid.” For example, HNO2 is nitrous acid.
Naming Bases
Bases are typically named by using the name of the cation followed by the word “hydroxide.” The cation is the positively charged ion that is formed when a base accepts a proton. For example, NaOH is sodium hydroxide, and KOH is potassium hydroxide.
Understanding the naming conventions for acids and bases allows chemists to communicate effectively and identify compounds accurately. By following these rules, scientists can easily recognize the nature and composition of various substances.
In conclusion, ammonia is classified as a base due to its ability to accept protons from acids. Its classification is based on the Bronsted-Lowry theory, which defines bases as substances that accept protons. Additionally, acids and bases have specific naming conventions that help differentiate them from other compounds. By understanding these naming rules, chemists can identify and categorize acids and bases accurately.
NH3 Lewis Acid or Base
Ammonia (NH3) is a fascinating compound that can exhibit both acidic and basic properties. In this section, we will explore the nature of ammonia as a Lewis base and delve into the theory of Lewis acids and bases.
Confirmation of Ammonia as a Lewis Base
Ammonia is commonly known as a weak base due to its ability to accept a proton (H+) from a donor molecule. This property allows ammonia to form a conjugate acid, ammonium ion (NH4+), in acid-base reactions. However, ammonia’s basicity extends beyond the traditional Bronsted-Lowry theory.
In the Lewis acid-base theory, a Lewis base is defined as a molecule or ion that donates a pair of electrons to form a covalent bond. Ammonia fits this definition perfectly as it possesses a lone pair of electrons on the nitrogen atom, which can be donated to an electron-deficient species, known as a Lewis acid.
Explanation of Lewis Acid and Base Theory
The Lewis acid-base theory, proposed by Gilbert N. Lewis in 1923, expands the concept of acids and bases beyond the traditional proton transfer. According to this theory, a Lewis acid is a substance that can accept a pair of electrons, while a Lewis base is a substance that can donate a pair of electrons.
In the case of ammonia, it acts as a Lewis base by donating its lone pair of electrons to a Lewis acid. This interaction forms a coordinate covalent bond, where the Lewis acid accepts the electron pair from the Lewis base. The resulting compound is called a Lewis adduct.
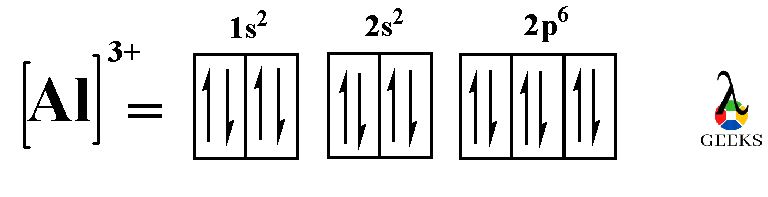
Lewis acids can be either neutral molecules or positively charged ions. Some common examples of Lewis acids include metal cations, such as aluminum (Al3+) and boron (B3+), as well as molecules like carbon dioxide (CO2) and hydrogen chloride (HCl).
The Lewis acid-base theory provides a more comprehensive understanding of chemical reactions, as it encompasses a broader range of interactions compared to the Bronsted-Lowry theory. It allows us to explain the formation of complex compounds and reactions that do not involve proton transfer.
To summarize, ammonia (NH3) is not only a weak base according to the Bronsted-Lowry theory but also a Lewis base according to the Lewis acid-base theory. Its ability to donate a pair of electrons makes it an essential participant in various chemical reactions.
In the next section, we will explore the acidic properties of ammonia and its role in the formation of ammonium ions. Stay tuned!
References:
– Lewis, G. N. (1923). “Valence and the Structure of Atoms and Molecules”. Chemical Catalog Company.
– Housecroft, C. E., & Sharpe, A. G. (2018). Inorganic Chemistry (5th ed.). Pearson.
NH3 Conjugate Acid or Base
Ammonia (NH3) is an interesting compound that exhibits the ability to act as both a conjugate acid and a conjugate base. This dual nature of ammonia allows it to participate in acid-base reactions and play a crucial role in various chemical processes.
Discussion on ammonia’s ability to act as both a conjugate acid and a conjugate base
Ammonia is a weak base, according to the Bronsted-Lowry theory of acids and bases. It can accept a proton (H+) from a stronger acid, making it a conjugate acid. On the other hand, ammonia can also donate a lone pair of electrons to form a coordinate bond with a Lewis acid, making it a Lewis base.
When ammonia acts as a base, it accepts a proton (H+) from a stronger acid, resulting in the formation of the ammonium ion (NH4+). This process can be represented by the following equation:
NH3 + H+ → NH4+
In this reaction, ammonia acts as a base by accepting a proton from the acid. The resulting species, NH4+, is the conjugate acid of ammonia.
Conversely, when ammonia acts as an acid, it donates a lone pair of electrons to a Lewis base, forming a coordinate bond. This can be illustrated by the reaction between ammonia and a hydroxide ion (OH-):
NH3 + OH- → NH2- + H2O
In this reaction, ammonia donates a lone pair of electrons to the hydroxide ion, forming the amide ion (NH2-) and water (H2O). Here, ammonia acts as an acid by donating a proton (H+) to the hydroxide ion, resulting in the formation of the amide ion.
Formation of NH4+ and NH2- ions
The formation of the ammonium ion (NH4+) and the amide ion (NH2-) demonstrates the dual nature of ammonia as both a conjugate acid and a conjugate base.
When ammonia accepts a proton (H+), it forms the ammonium ion (NH4+). This process occurs in acidic solutions where there is an excess of H+ ions available. The ammonium ion is a positively charged species and can further participate in chemical reactions.
On the other hand, when ammonia donates a proton (H+), it forms the amide ion (NH2-). This process occurs in basic solutions where there is an excess of OH- ions available. The amide ion is a negatively charged species and can also participate in various chemical reactions.
In summary, ammonia’s ability to act as both a conjugate acid and a conjugate base allows it to participate in acid-base reactions and play a vital role in various chemical processes. Its dual nature makes it a versatile compound with a wide range of applications in industries such as agriculture, pharmaceuticals, and manufacturing.
What is the Conjugate Acid and Base of NH3?
Ammonia (NH3) is a compound that can act as both an acid and a base, depending on the context. When ammonia reacts with an acid, it acts as a base, accepting a proton (H+) to form its conjugate acid. On the other hand, when ammonia reacts with a base, it acts as an acid, donating a proton to form its conjugate base.
Identification of NH4+ as the conjugate acid of NH3
When ammonia (NH3) reacts with an acid, it accepts a proton (H+) to form its conjugate acid, which is called ammonium ion (NH4+). This reaction can be represented as follows:
NH3 + H+ → NH4+
In this reaction, ammonia acts as a base by accepting a proton from the acid. The resulting ammonium ion (NH4+) is the conjugate acid of ammonia.
Identification of NH2- as the conjugate base of NH3
When ammonia (NH3) reacts with a base, it donates a proton to form its conjugate base, which is called amide ion (NH2-). This reaction can be represented as follows:
NH3 + B- → NH2- + HB
In this reaction, ammonia acts as an acid by donating a proton to the base. The resulting amide ion (NH2-) is the conjugate base of ammonia.
It is important to note that the conjugate acid and base of a compound are always related to each other. In the case of ammonia, its conjugate acid (ammonium ion) and conjugate base (amide ion) differ by the presence or absence of a proton.
By understanding the nature of ammonia as both an acid and a base, we can appreciate its versatility in various chemical reactions. Whether it is reacting with an acid or a base, ammonia can adapt and participate in the formation of its conjugate acid or base, respectively. This ability to act as both an acid and a base is a characteristic of amphoteric compounds, which adds to the significance of ammonia in chemistry.
In the next section, we will explore the acid-base properties of ammonia in more detail and discuss its role in pH regulation and other chemical reactions.
NH3 Acid, Base, or Salt?
Explanation of ammonia as a base rather than an acid or salt
Ammonia (NH3) is commonly known as a base rather than an acid or salt. But what exactly does that mean? Let’s dive into the nature of ammonia and understand why it is classified as a base.
Understanding Ammonia
Ammonia is a compound composed of one nitrogen atom bonded with three hydrogen atoms. It is a colorless gas with a pungent odor and is commonly used in cleaning products, fertilizers, and various industrial processes. In its pure form, ammonia readily dissolves in water to form ammonium hydroxide (NH4OH), a solution commonly known as ammonia water.
Bronsted-Lowry Theory
To understand why ammonia is considered a base, we need to explore the Bronsted-Lowry theory of acids and bases. According to this theory, an acid is a substance that donates a proton (H+) to another substance, while a base is a substance that accepts a proton.
In the case of ammonia, it acts as a base because it can accept a proton from an acid. When ammonia reacts with water, it forms ammonium ions (NH4+) and hydroxide ions (OH-). The ammonia molecule accepts a proton from a water molecule, resulting in the formation of ammonium ions and hydroxide ions.
Ammonia as a Lewis Base
In addition to being a Bronsted-Lowry base, ammonia can also act as a Lewis base. The Lewis theory of acids and bases focuses on electron pair donation. In this theory, a Lewis acid is a substance that accepts an electron pair, while a Lewis base is a substance that donates an electron pair.
Ammonia acts as a Lewis base by donating its lone pair of electrons to a Lewis acid. This electron pair donation forms a coordinate covalent bond between the ammonia molecule and the Lewis acid.
Properties of Ammonia as a Base
As a base, ammonia exhibits certain properties. It has a bitter taste and feels slippery to the touch. Ammonia can also neutralize acids, forming salts in the process. For example, when ammonia reacts with hydrochloric acid (HCl), it forms ammonium chloride (NH4Cl), a salt.
Ammonia is considered a weak base because it does not completely dissociate in water. It only partially accepts protons, resulting in a limited concentration of hydroxide ions in the solution. This is in contrast to strong bases like sodium hydroxide (NaOH), which completely dissociate to produce a high concentration of hydroxide ions.
Applications of Ammonia as a Base
Ammonia’s basic properties make it useful in various applications. It is commonly used in household cleaning products, such as window cleaners and floor cleaners, due to its ability to neutralize acidic substances effectively.
In the field of agriculture, ammonia is a key component of fertilizers. It provides essential nitrogen to plants, promoting their growth and development. Ammonia is also used in the production of various chemicals, including plastics, dyes, and pharmaceuticals.
In conclusion, ammonia is classified as a base due to its ability to accept protons from acids and donate electron pairs to Lewis acids. Its basic properties and applications make it a versatile compound with various uses in different industries. Understanding the nature of ammonia as a base helps us appreciate its role in our daily lives and its significance in chemical processes.
Ammonia Acid or Base in Water
Discussion on Ammonia’s Behavior in Water
When it comes to discussing ammonia’s behavior in water, it is important to understand its nature as an acid or a base. Ammonia, with the chemical formula NH3, is a compound that consists of one nitrogen atom and three hydrogen atoms. It is commonly used in various industries, including agriculture, cleaning products, and refrigeration.
In water, ammonia exhibits properties of both an acid and a base. This dual behavior is due to its ability to donate and accept protons, which are positively charged particles. Let’s delve deeper into how ammonia behaves in water and the formation of NH4+ and OH- ions.
Formation of NH4+ and OH- Ions
When ammonia is dissolved in water, it undergoes an acid-base reaction. The water molecules act as a solvent and surround the ammonia molecules, causing them to dissociate. This dissociation leads to the formation of NH4+ and OH- ions.
Ammonia, being a weak base, accepts a proton (H+) from a water molecule, resulting in the formation of the ammonium ion (NH4+). This process is known as protonation. The ammonium ion is a positively charged species and is responsible for the acidic behavior of ammonia in water.
On the other hand, the water molecule that donates the proton to ammonia becomes a hydroxide ion (OH-). The hydroxide ion is a negatively charged species and is responsible for the basic behavior of ammonia in water.
The equilibrium between ammonia, ammonium ion, and hydroxide ion can be represented by the following chemical equation:
NH3 + H2O ⇌ NH4+ + OH-
In this equation, the double-headed arrow indicates that the reaction can proceed in both directions. This means that ammonia can act as both an acid and a base simultaneously in water.
It is worth noting that the extent to which ammonia behaves as an acid or a base depends on its concentration in the water. At low concentrations, ammonia primarily acts as a base, while at higher concentrations, it tends to exhibit more acidic behavior.
In summary, ammonia exhibits both acidic and basic behavior in water. It can accept a proton from a water molecule to form the ammonium ion, making it an acid. Simultaneously, it can donate a proton to a water molecule, resulting in the formation of the hydroxide ion, making it a base. This dual nature of ammonia makes it a fascinating compound with diverse applications in various industries.
NH3 + H2O Acid or Base?
Explanation of ammonia’s behavior when added to water
When ammonia (NH3) is added to water (H2O), it undergoes an acid-base reaction. This reaction is fascinating because it showcases the unique properties of ammonia as both an acid and a base.
Ammonia is a compound that consists of one nitrogen atom bonded to three hydrogen atoms. It is commonly used in household cleaning products and as a fertilizer. In its pure form, ammonia is a colorless gas with a pungent odor.
When ammonia is added to water, it readily dissolves due to its polar nature. The water molecules surround the ammonia molecules, breaking the hydrogen bonds between the ammonia molecules and allowing them to mix with the water.
Formation of NH4+ and OH- ions
The acid-base reaction between ammonia and water results in the formation of two ions: the ammonium ion (NH4+) and the hydroxide ion (OH-).
Ammonia acts as a base in this reaction by accepting a proton (H+) from water. The lone pair of electrons on the nitrogen atom in ammonia attracts the proton from water, forming the ammonium ion (NH4+). This process is known as protonation.
On the other hand, water acts as an acid by donating a proton to ammonia. The water molecule donates a proton to the lone pair of electrons on the nitrogen atom in ammonia, forming the hydroxide ion (OH-). This process is known as deprotonation.
The formation of the ammonium ion and the hydroxide ion in the presence of water leads to the creation of an alkaline solution. The concentration of hydroxide ions determines the pH of the solution. A higher concentration of hydroxide ions results in a higher pH, indicating a more basic solution.
In summary, when ammonia is added to water, it behaves as both an acid and a base. It accepts a proton from water, forming the ammonium ion, and donates a proton to water, forming the hydroxide ion. This acid-base reaction results in the formation of an alkaline solution.
NH3 Arrhenius Acid or Base
Explanation of ammonia as an Arrhenius base
Ammonia (NH3) is a compound that can act as both an acid and a base, depending on the context in which it is used. In the Arrhenius theory of acids and bases, an acid is defined as a substance that produces hydrogen ions (H+) when dissolved in water, while a base is a substance that produces hydroxide ions (OH-) when dissolved in water.
Ammonia, in its pure form, is a weak base. When it is dissolved in water, it can accept a proton (H+) from water molecules, forming ammonium ions (NH4+) and hydroxide ions (OH-). This reaction can be represented as follows:
NH3 + H2O ⇌ NH4+ + OH-
In this reaction, ammonia acts as a base by accepting a proton from water, resulting in the formation of hydroxide ions. The hydroxide ions give the solution basic properties.
Formation of OH- ions in aqueous solution
When ammonia is dissolved in water, it undergoes a reaction with water molecules to produce hydroxide ions (OH-). This reaction is known as the hydrolysis of ammonia. The hydrolysis reaction can be represented as follows:
NH3 + H2O ⇌ NH4+ + OH-
In this reaction, ammonia acts as a base by accepting a proton (H+) from water, forming ammonium ions (NH4+) and hydroxide ions (OH-). The hydroxide ions give the solution basic properties.
It is important to note that the extent to which ammonia acts as a base depends on its concentration in the solution. Higher concentrations of ammonia will result in a greater production of hydroxide ions, making the solution more basic.
In summary, ammonia (NH3) can act as a base in aqueous solutions by accepting a proton from water, resulting in the formation of hydroxide ions (OH-). This property of ammonia makes it an important component in many chemical reactions and industrial processes.
What is Ammonia Acid or Base?
Ammonia, also known as NH3, is a compound that can act as both an acid and a base. In chemistry, substances are classified as acids or bases based on their ability to donate or accept protons (H+ ions). Ammonia is an interesting molecule because it can exhibit both acidic and basic properties, depending on the context.
Recap of Ammonia’s Classification as a Base
Ammonia is commonly referred to as a base due to its ability to accept protons. According to the Brønsted-Lowry theory, a base is a substance that can accept a proton. In the case of ammonia, it can accept a proton to form the ammonium ion (NH4+). This reaction can be represented as follows:
NH3 + H+ → NH4+
By accepting a proton, ammonia acts as a base and forms its conjugate acid, the ammonium ion. The ammonium ion is a positively charged species that is formed when ammonia gains a proton.
Explanation of its Ability to Increase OH- Ion Concentration
One of the key characteristics of bases is their ability to increase the concentration of hydroxide ions (OH-) in a solution. When ammonia dissolves in water, it can react with water molecules to produce hydroxide ions. This reaction is known as the hydrolysis of ammonia:
NH3 + H2O ⇌ NH4+ + OH-
In this reaction, ammonia acts as a base by accepting a proton from water, forming the ammonium ion (NH4+) and hydroxide ion (OH-). The hydroxide ion is responsible for the basic properties of ammonia.
It’s important to note that ammonia is a weak base, meaning it does not completely dissociate in water and only a small fraction of ammonia molecules react with water to form the ammonium ion and hydroxide ion. This is in contrast to strong bases, which completely dissociate in water and produce a high concentration of hydroxide ions.
In summary, ammonia can be classified as a base due to its ability to accept protons and increase the concentration of hydroxide ions in a solution. Its basic properties make it a versatile compound with various applications in industries such as agriculture, cleaning products, and refrigeration.
Is NH3 a Bronsted Base?
The classification of NH3 as a Bronsted base can be confirmed through the application of the Bronsted-Lowry theory. This theory, proposed by Johannes Nicolaus Bronsted and Thomas Martin Lowry, provides a comprehensive framework for understanding acid-base reactions.
Confirmation of ammonia as a Bronsted base
According to the Bronsted-Lowry theory, a Bronsted base is a substance that can accept a proton (H+) from another substance. In the case of NH3, it can act as a base by accepting a proton from an acid. When NH3 accepts a proton, it forms the ammonium ion (NH4+), which is the conjugate acid of ammonia.
Ammonia, with its lone pair of electrons, readily forms a coordinate bond with a proton, resulting in the formation of the ammonium ion. This reaction can be represented as follows:
NH3 + H+ → NH4+
Explanation of Bronsted-Lowry theory
The Bronsted-Lowry theory expands upon the concept of acids and bases beyond the traditional definition of substances that release or accept hydrogen ions (H+). According to this theory, an acid is a substance that donates a proton, while a base is a substance that accepts a proton.
In the context of the Bronsted-Lowry theory, a proton refers to a hydrogen ion (H+). Acids and bases are considered to be conjugate pairs, where an acid and its corresponding base differ by the presence or absence of a proton. For example, in the reaction between NH3 and H+, NH3 acts as a base by accepting a proton to form NH4+.
It is important to note that the strength of an acid or base is not determined solely by its ability to donate or accept protons. The strength of an acid or base is influenced by factors such as the stability of the resulting conjugate base or acid and the polarity of the molecule.
In summary, NH3 can be classified as a Bronsted base based on its ability to accept a proton from an acid. The Bronsted-Lowry theory provides a comprehensive framework for understanding acid-base reactions, allowing us to identify substances as acids or bases based on their proton-donating or proton-accepting abilities.
Why is NH3 a Weak Bronsted Base?
Ammonia (NH3) is classified as a weak Bronsted base due to its unique chemical properties. Let’s explore the reasons behind its weak base nature.
Explanation of ammonia’s weak base nature
Ammonia is a compound composed of one nitrogen atom bonded to three hydrogen atoms. In an acid-base reaction, ammonia acts as a base by accepting a proton (H+) from an acid. However, compared to other bases, ammonia has a limited ability to accept protons.
One key factor contributing to ammonia’s weak base nature is its low affinity for protons. The nitrogen atom in ammonia has a lone pair of electrons, which can be donated to form a bond with a proton. However, this lone pair is not as readily available for donation as in stronger bases. As a result, ammonia has a limited capacity to accept protons and is considered a weak base.
Limited production of OH- ions in aqueous solution
When ammonia is dissolved in water, it undergoes a reaction with water molecules to produce ammonium ions (NH4+) and hydroxide ions (OH-). This reaction is known as the hydrolysis of ammonia.
However, the production of hydroxide ions in this reaction is relatively low compared to strong bases. This is because ammonia is a weak base and does not readily donate its lone pair of electrons to form hydroxide ions. Instead, it primarily forms ammonium ions by accepting a proton from water.
The equilibrium between ammonia, water, ammonium ions, and hydroxide ions lies more towards the formation of ammonium ions. As a result, the concentration of hydroxide ions in an aqueous solution of ammonia is relatively low, further supporting its classification as a weak base.
In summary, ammonia’s weak base nature can be attributed to its limited ability to accept protons and the relatively low production of hydroxide ions in aqueous solution. While it can still participate in acid-base reactions, it does so to a lesser extent compared to stronger bases.
| Key Points |
| – Ammonia is a weak Bronsted base due to its low affinity for protons. |
| – The limited production of hydroxide ions in aqueous solution contributes to ammonia’s weak base nature. |
| – Ammonia primarily forms ammonium ions when dissolved in water. |
Is NH3 a Conjugate Acid or Base?
Ammonia (NH3) is a fascinating compound that exhibits behavior as both a conjugate acid and a conjugate base. Let’s delve into the explanation of ammonia’s behavior in these roles.
Explanation of Ammonia’s Behavior as Both a Conjugate Acid and a Conjugate Base
Ammonia, with its chemical formula NH3, is a compound composed of one nitrogen atom bonded to three hydrogen atoms. It is a versatile molecule that can participate in acid-base reactions, showcasing its dual nature.
Ammonia as a Conjugate Acid
In an acid-base reaction, ammonia can act as a conjugate acid. A conjugate acid is formed when a base accepts a proton (H+) from an acid. In this case, ammonia accepts a proton to form its conjugate acid, the ammonium ion (NH4+). This reaction can be represented as follows:
NH3 + H+ → NH4+
Here, ammonia (NH3) accepts a proton (H+) to become the ammonium ion (NH4+). The ammonium ion is positively charged due to the addition of the proton.
Ammonia as a Conjugate Base
On the other hand, ammonia can also act as a conjugate base. A conjugate base is formed when an acid donates a proton (H+) to a base. In this scenario, ammonia acts as a base by accepting a proton from an acid to form its conjugate base, the amide ion (NH2-). The reaction can be represented as:
NH3 + H+ → NH2-
In this reaction, ammonia (NH3) accepts a proton (H+) from an acid, resulting in the formation of the amide ion (NH2-). The amide ion carries a negative charge due to the loss of the proton.
Ammonia’s ability to act as both a conjugate acid and a conjugate base is a result of its unique molecular structure and the presence of a lone pair of electrons on the nitrogen atom. This lone pair of electrons allows ammonia to readily accept or donate a proton, depending on the reaction conditions.
It is important to note that ammonia is considered a weak base in aqueous solutions. This means that it does not completely dissociate into hydroxide ions (OH-) like strong bases such as sodium hydroxide (NaOH). Instead, only a small fraction of ammonia molecules react with water to produce ammonium ions (NH4+) and hydroxide ions (OH-). The equilibrium between ammonia and its conjugate acid, the ammonium ion, is established in this process.
In summary, ammonia (NH3) demonstrates behavior as both a conjugate acid and a conjugate base. Its ability to accept or donate a proton allows it to participate in acid-base reactions, making it a versatile compound in various chemical processes.
Conclusion
In conclusion, ammonia (NH3) is a fascinating compound that can exhibit both acidic and basic properties depending on the context. While it is technically a weak base due to its ability to accept a proton, it can also act as a weak acid by donating a lone pair of electrons. This dual nature of ammonia makes it a versatile compound with a wide range of applications in various industries. Whether it’s used as a cleaning agent, a refrigerant, or a precursor in the production of fertilizers, ammonia plays a crucial role in our daily lives. Understanding its acid-base behavior is essential for scientists and chemists alike, as it helps us comprehend the complex interactions between different substances. So, the next time you encounter ammonia, remember that it can be both an acid and a base, showcasing the fascinating nature of chemistry.
Frequently Asked Questions
1. Is ammonia acidic, neutral, or basic?
Ammonia (NH3) is a basic substance.
2. Is NH3 a good base?
Yes, NH3 is a good base.
3. Is NH3 an acid or base? Is it strong or weak?
NH3 is a base, and it is a weak base.
4. What is NH3? Is it an acid or base?
NH3 is ammonia, which is a base.
5. Is NH3 acidic or basic?
NH3 is basic in nature.
6. Why does NH3 act as a base?
NH3 acts as a base because it can accept a proton (H+) to form the ammonium ion (NH4+).
7. Is NH3 (aq) an acid or base?
NH3 (aq) is a base when dissolved in water.
8. Is NH3 acidic, basic, or neutral?
NH3 is a basic substance.
9. What is the name of NH3 as an acid or base?
NH3 is commonly known as ammonia, which is a base.
10. Is NH3 a Lewis acid or base?
NH3 is a Lewis base because it can donate a pair of electrons to form a coordinate bond with a Lewis acid.
11. What is the conjugate acid and base of NH3?
The conjugate acid of NH3 is NH4+ (ammonium ion), and the conjugate base is NH2- (amide ion).
12. Is NH3 an acid, base, or salt?
NH3 is a base, not an acid or salt.
13. Is ammonia an acid or base in water?
Ammonia (NH3) acts as a base when dissolved in water.
14. Is NH3 + H2O an acid or base?
NH3 + H2O acts as a base in an acid-base reaction.
15. Is NH3 an Arrhenius acid or base?
NH3 is not an Arrhenius acid or base. It is a Lewis base.
16. What is ammonia? Is it an acid or base?
Ammonia (NH3) is a compound that acts as a base in acid-base reactions.