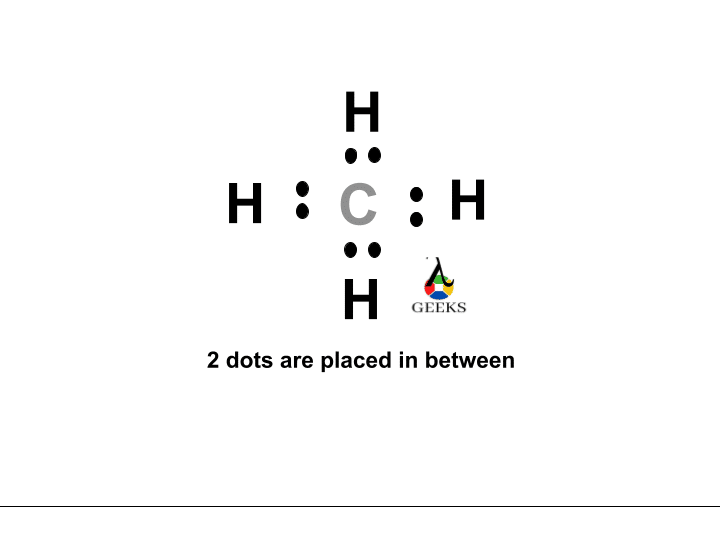
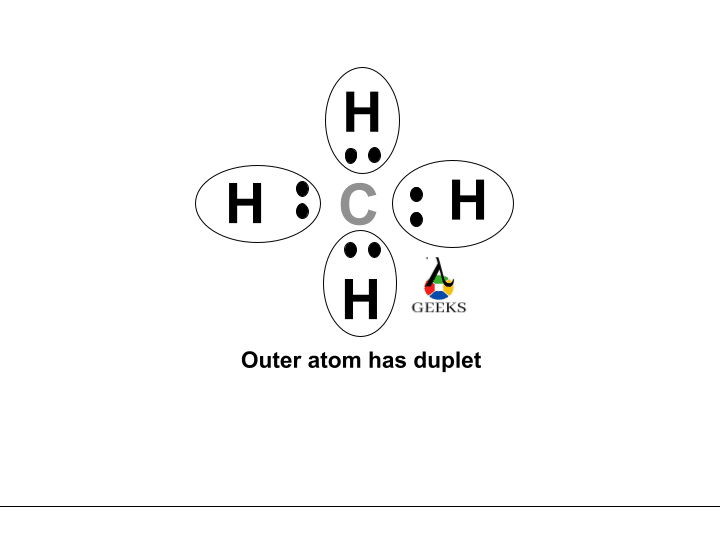
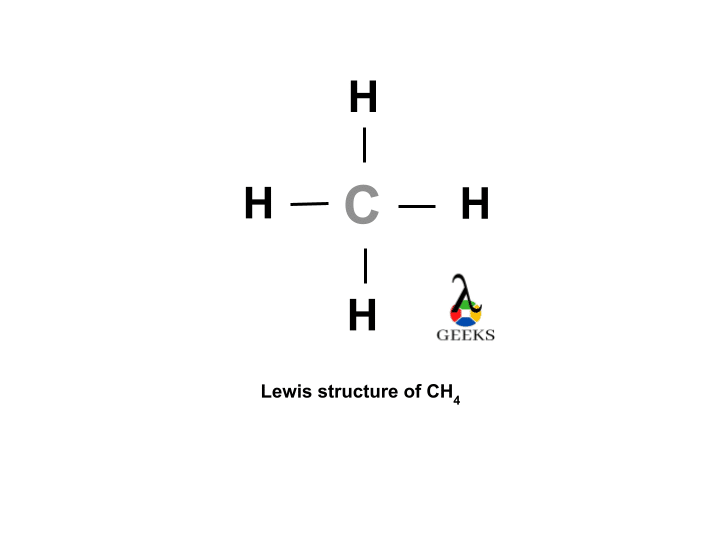
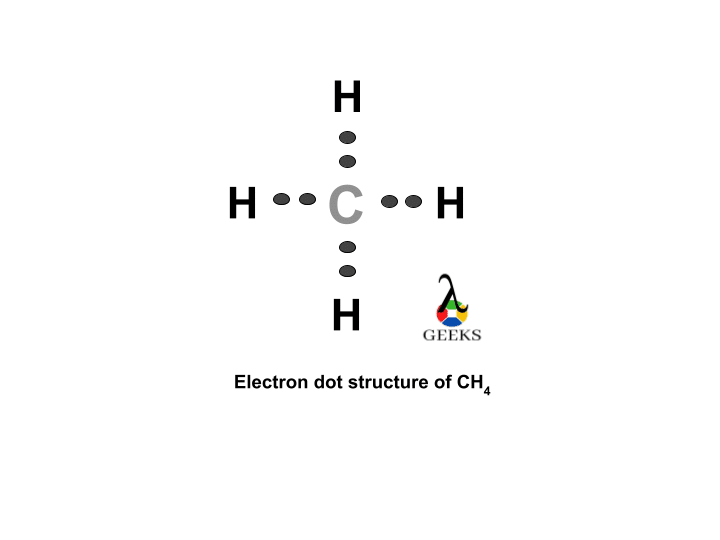
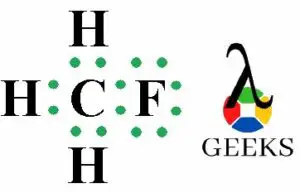
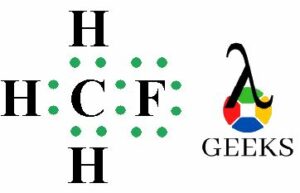
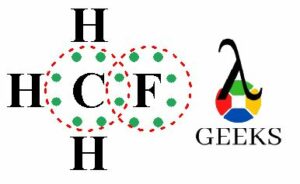
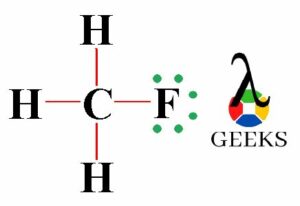
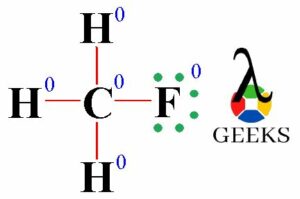
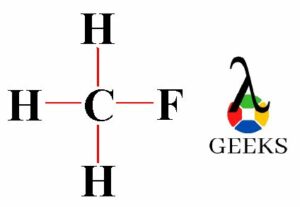
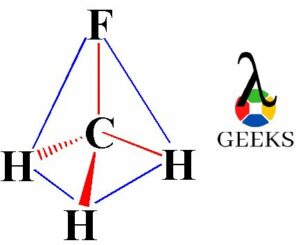
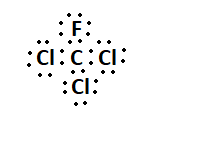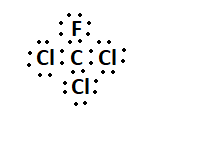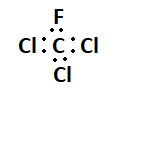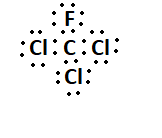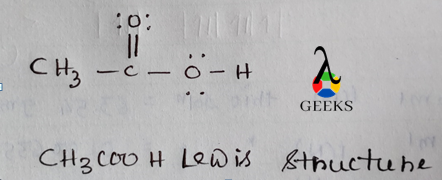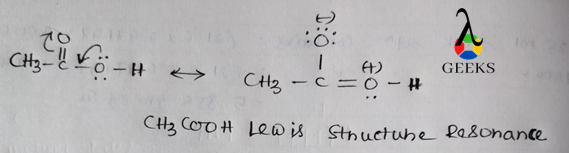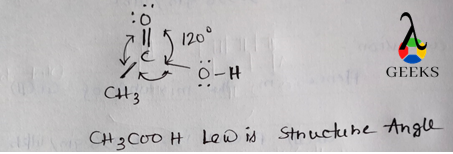The ClF (Chlorine monofluoride) has a simple linear Lewis structure: a single bond between chlorine (Cl) and fluorine (F), each with 7 valence electrons. Total of 14 valence electrons are used. No lone pairs on the Cl-F bond, resulting in a 180° bond angle. Electronegativity values: Cl (3.16), F (3.98), showing a significant difference, indicating a polar covalent bond. The molecule is polar due to this asymmetry in electronegativity, despite its linear geometry.
Understanding ClF Lewis Structure
The ClF Lewis structure refers to the representation of the chemical bonding and electron distribution in a molecule of chlorine fluoride (ClF). By understanding the Lewis structure of ClF, we can gain insights into its molecular geometry, electron configuration, and chemical reactivity.

How to Draw ClF Lewis Structure

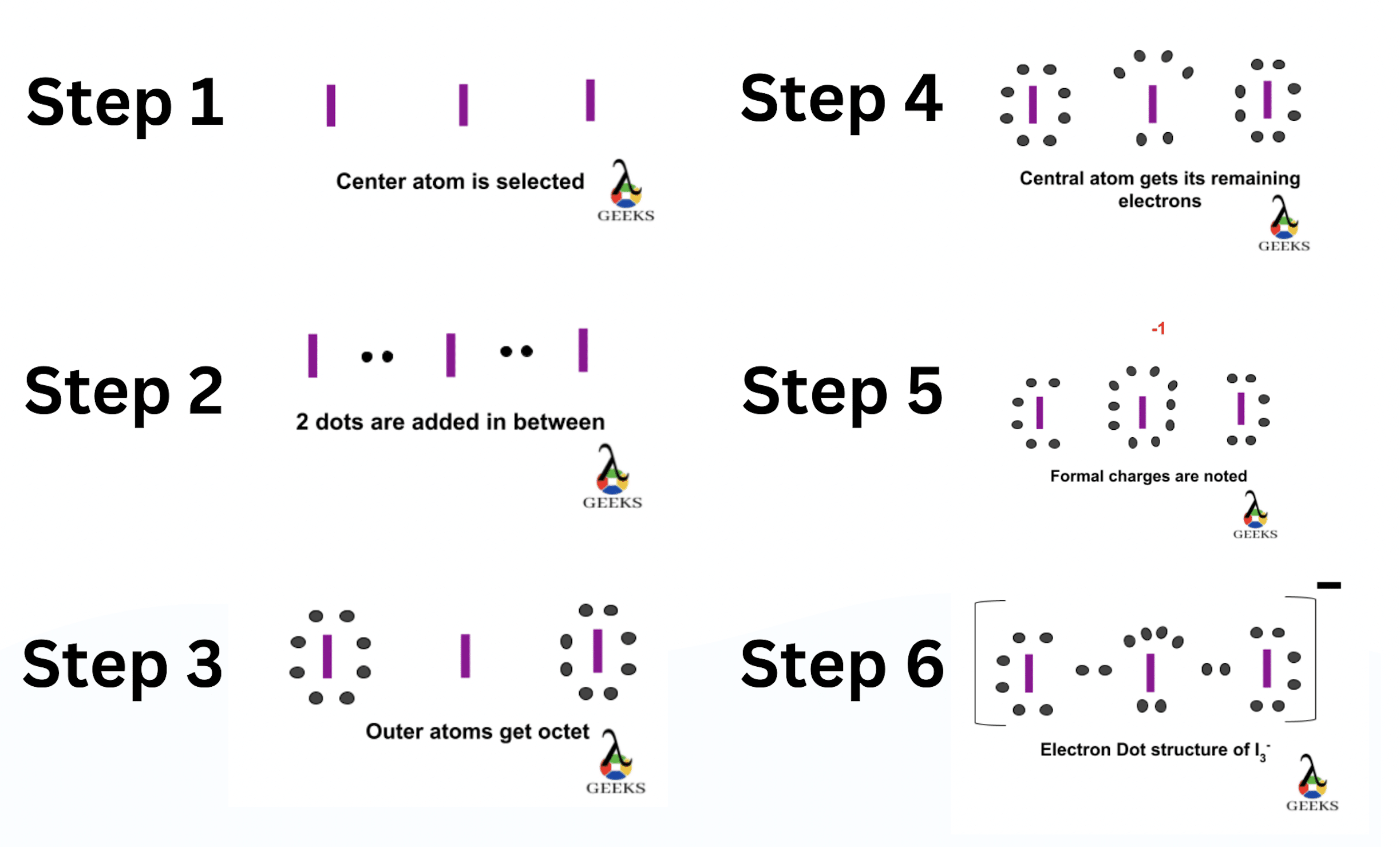
To draw the Lewis dot diagram for ClF, we need to follow a few steps:
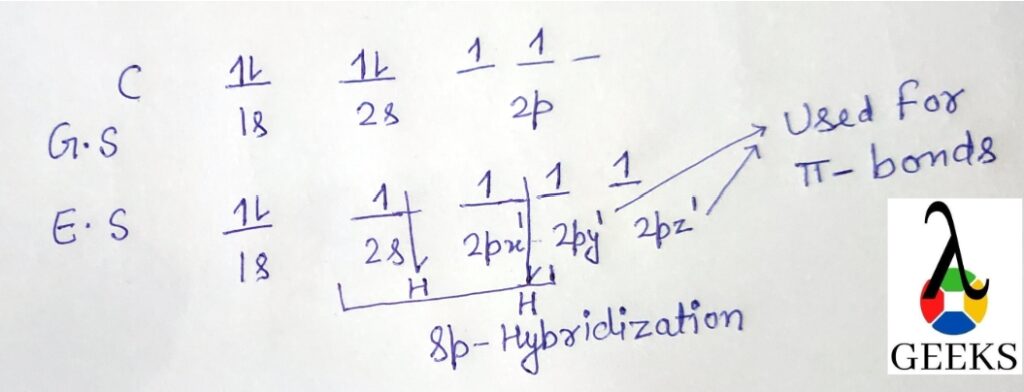
- Determine the total number of valence electrons in ClF. Chlorine (Cl) is in Group 7A of the periodic table and has 7 valence electrons, while fluorine (F) is in Group 7A and also has 7 valence electrons. Therefore, the total number of valence electrons in ClF is 7 + 7 = 14.
- Identify the central atom. In ClF, chlorine (Cl) is the central atom since it is less electronegative than fluorine (F).
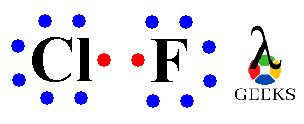
- Connect the central atom (Cl) to the surrounding atoms (F) using single bonds. In this case, ClF will have one chlorine atom bonded to one fluorine atom.
- Distribute the remaining valence electrons around the atoms to satisfy the octet rule. The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with 8 valence electrons. In ClF, we have 14 valence electrons to distribute. We place 2 electrons as a lone pair on the chlorine atom and 6 electrons as lone pairs on the fluorine atom.
- Check if all atoms have achieved an octet. In ClF, both the chlorine and fluorine atoms have 8 valence electrons, satisfying the octet rule.
The Lewis structure of ClF can be represented as Cl:F with a single bond between the chlorine and fluorine atoms.
ClF Valence Electrons
Valence electrons are the electrons in the outermost energy level of an atom. In the case of ClF, chlorine (Cl) and fluorine (F) both belong to Group 7A of the periodic table, which means they have 7 valence electrons each. Therefore, the total number of valence electrons in ClF is 7 + 7 = 14.
ClF Lewis Structure Octet Rule

The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with 8 valence electrons. In the Lewis structure of ClF, both the chlorine and fluorine atoms have 8 valence electrons, satisfying the octet rule. The chlorine atom achieves an octet by sharing one electron with the fluorine atom through a single bond.
ClF Lewis Structure Lone Pairs
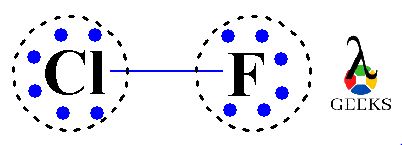
Lone pairs are pairs of valence electrons that are not involved in bonding. In the Lewis structure of ClF, we have 2 lone pairs of electrons on the chlorine atom and 6 lone pairs of electrons on the fluorine atom. These lone pairs contribute to the overall electron distribution and molecular geometry of ClF.
ClF Lewis Structure Formal Charge
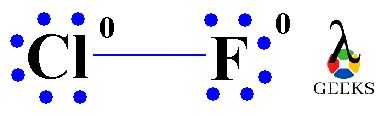
Formal charge is a concept used to determine the distribution of electrons in a molecule or ion. It helps us understand the stability and reactivity of the compound. In the Lewis structure of ClF, the chlorine atom has a formal charge of 0, while the fluorine atom also has a formal charge of 0. This indicates that the electron distribution in ClF is balanced and stable.
ClF Lewis Structure Resonance

Resonance structures are alternative Lewis structures that represent the delocalization of electrons within a molecule. In the case of ClF, there are no resonance structures since the distribution of electrons is fixed and does not vary between different arrangements.
By understanding the ClF Lewis structure, we can gain insights into its molecular geometry, electron configuration, and chemical reactivity. The Lewis structure provides a visual representation of the chemical bonding and electron distribution in ClF, allowing us to analyze its properties and behavior.
Properties of ClF Lewis Structure
ClF Lewis Structure Shape
The Lewis structure of ClF (chlorine fluoride) is a representation of the chemical bonding between chlorine and fluorine atoms. It is a covalent compound, meaning that the atoms share electrons to form bonds. In the Lewis dot diagram, the chlorine atom is surrounded by one lone pair of electrons and is bonded to a single fluorine atom. This arrangement gives ClF a linear molecular geometry, with the chlorine atom at the center and the fluorine atom on one side.
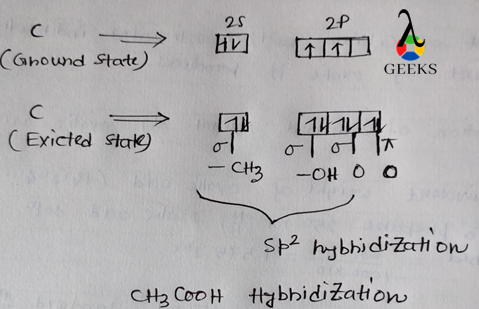
ClF Hybridization
The hybridization of ClF is sp. Hybridization refers to the mixing of atomic orbitals to form new hybrid orbitals that are used for bonding. In the case of ClF, the chlorine atom undergoes sp hybridization, which means that one s orbital and one p orbital combine to form two sp hybrid orbitals. These hybrid orbitals then overlap with the fluorine atom’s 2p orbital to form a sigma bond.
ClF Lewis Structure Angle
The linear molecular geometry of ClF gives it a bond angle of 180 degrees. This angle is determined by the repulsion between the lone pair of electrons on the chlorine atom and the bonding pair of electrons between chlorine and fluorine. According to the VSEPR theory (Valence Shell Electron Pair Repulsion theory), the lone pair exerts greater repulsion than the bonding pair, causing the molecule to adopt a linear shape.
ClF Solubility
ClF is a polar molecule due to the difference in electronegativity between chlorine and fluorine. This polarity makes ClF soluble in polar solvents such as water. When ClF is dissolved in water, the polar water molecules interact with the polar ClF molecules, resulting in the formation of hydrogen bonds. These hydrogen bonds help to stabilize the ClF molecules in the solution.
Is ClF Ionic or Covalent?
ClF is a covalent compound rather than an ionic compound. In covalent bonds, atoms share electrons to achieve a stable electron configuration. In the case of ClF, the chlorine and fluorine atoms share electrons to form a covalent bond. Ionic compounds, on the other hand, involve the transfer of electrons from one atom to another, resulting in the formation of ions. ClF does not exhibit this characteristic, making it a covalent compound.
Chemical Behavior of ClF
Chlorine fluoride (ClF) is a chemical compound that exhibits interesting chemical behavior. Let’s explore some key aspects of its behavior.
Is ClF Acidic or Basic?
When it comes to acidity or basicity, ClF is considered an acidic compound. This is because it can donate a proton (H+) in a chemical reaction. The presence of a lone pair of electrons on the chlorine atom allows it to act as a Lewis acid, accepting an electron pair from a Lewis base.
Is ClF Polar or Nonpolar?
ClF is a polar molecule due to the difference in electronegativity between chlorine and fluorine atoms. Chlorine is more electronegative than fluorine, resulting in an uneven distribution of electron density within the molecule. This creates a partial positive charge on the chlorine atom and a partial negative charge on the fluorine atom, leading to a polar bond.
Is ClF Tetrahedral?
No, ClF is not tetrahedral in shape. The molecular geometry of ClF is bent or V-shaped. This is because of the presence of two electron pairs around the central chlorine atom. According to the VSEPR theory (Valence Shell Electron Pair Repulsion theory), the repulsion between these electron pairs causes the molecule to adopt a bent shape.
Is ClF Linear?
No, ClF is not linear in shape. As mentioned earlier, ClF has a bent molecular geometry. In a linear molecule, the atoms are arranged in a straight line, but in ClF, the presence of lone pairs and the repulsion between electron pairs cause the molecule to deviate from linearity.
Advanced Concepts in ClF Lewis Structure
Chlorine monofluoride (ClF) is a chemical compound that exhibits interesting properties in its Lewis structure. By understanding its molecular geometry, polarity, and various Lewis structures, we can gain insights into its chemical bonding and reactivity.
ClF Lewis Structure Molecular Geometry
The molecular geometry of ClF is determined by the arrangement of its atoms and electron pairs. In the case of ClF, there are two regions of electron density around the central chlorine atom. This leads to a linear molecular geometry, with the chlorine atom at the center and the fluorine atom on one side.
Lewis Structure ClF4-
When ClF gains an extra electron to form the ClF4- ion, the Lewis structure changes. The central chlorine atom now has four regions of electron density, resulting in a tetrahedral molecular geometry. The four fluorine atoms are arranged around the chlorine atom, each forming a covalent bond.
ClF Lewis Structure Polar or Nonpolar
The polarity of a molecule is determined by the presence of polar bonds and the overall molecular geometry. In the case of ClF, the chlorine-fluorine bond is polar due to the difference in electronegativity between the two atoms. However, since ClF has a linear molecular geometry, the polar bonds cancel each other out, resulting in a nonpolar molecule.
ClF-4 Lewis Structure
The Lewis structure of ClF-4, as mentioned earlier, has a tetrahedral molecular geometry. The central chlorine atom is surrounded by four fluorine atoms, each forming a covalent bond. The negative charge on the ion is represented by an additional electron pair.
ClF5 Lewis Structure
When an additional fluorine atom is added to ClF, the Lewis structure changes once again. The central chlorine atom now has five regions of electron density, resulting in a trigonal bipyramidal molecular geometry. The five fluorine atoms are arranged around the chlorine atom, forming covalent bonds.
ClF+2 Lewis Structure
In the case of ClF+2, the Lewis structure is similar to ClF, but with a positive charge on the molecule. The central chlorine atom has two regions of electron density, resulting in a linear molecular geometry. The positive charge is represented by the loss of one electron.
By exploring these various Lewis structures and molecular geometries, we can better understand the chemical bonding and behavior of ClF and its related compounds. These concepts are essential in studying chemical reactivity, molecular models, and the properties of different chemical compounds.
Remember to consider the valence electrons, octet rule, lone pairs, resonance structures, VSEPR theory, electron configuration, atomic orbitals, bond angles, polar and nonpolar molecules, hybridization, chemical notation, molecular shape, Lewis symbols, and atomic structure when analyzing ClF Lewis structures.
Practical Applications of ClF Lewis Structure
How to Determine if a Lewis Structure is Polar
When studying chemical bonding and molecular structure, understanding the polarity of a molecule is crucial. The Lewis structure, also known as the Lewis dot diagram, provides a visual representation of the covalent bonds and electron pairs in a molecule. By examining the Lewis structure, we can determine if a molecule is polar or nonpolar.
To determine if a Lewis structure is polar, we need to consider the arrangement of atoms and the distribution of electrons. If a molecule has polar bonds and an asymmetrical molecular geometry, it will be a polar molecule. On the other hand, if a molecule has nonpolar bonds or a symmetrical molecular geometry, it will be a nonpolar molecule.
In the case of ClF (chlorine trifluoride), the Lewis structure reveals that it has polar bonds due to the difference in electronegativity between chlorine and fluorine. However, the molecule’s T-shaped molecular geometry, which results from the presence of three bonding pairs and two lone pairs of electrons, makes it asymmetrical. Therefore, ClF is a polar molecule.
Why is ClF3 T-Shaped?
The T-shaped molecular geometry of ClF3 can be explained using the Valence Shell Electron Pair Repulsion (VSEPR) theory. According to this theory, electron pairs, whether bonding or nonbonding, repel each other and try to maximize their separation to minimize repulsion.
In the case of ClF3, there are three bonding pairs and two lone pairs of electrons around the central chlorine atom. The repulsion between these electron pairs causes the molecule to adopt a T-shaped geometry. The three bonding pairs form a trigonal plane, while the two lone pairs occupy the axial positions, resulting in the T-shaped arrangement.
Why Does ClF3 Have a Large Dipole?
The large dipole moment of ClF3 can be attributed to the molecule’s polar bonds and its T-shaped molecular geometry. A dipole moment is a measure of the separation of positive and negative charges within a molecule.
In ClF3, the chlorine atom is more electronegative than the fluorine atoms, resulting in polar bonds. The asymmetrical T-shaped geometry causes an uneven distribution of electron density, with the chlorine atom being partially negative and the fluorine atoms being partially positive. This separation of charges creates a large dipole moment in the molecule.
The practical applications of understanding the ClF Lewis structure, its polarity, and molecular geometry are significant. This knowledge helps in predicting the chemical reactivity, physical properties, and behavior of ClF3 and other similar chemical compounds. It also aids in the interpretation of experimental data and the design of molecular models for various applications.
By utilizing the principles of chemical bonding, Lewis structures, and molecular geometry, scientists and researchers can gain insights into the behavior and properties of different chemical compounds, contributing to advancements in fields such as materials science, pharmaceuticals, and environmental studies.
References
Credible Sources for Further Reading
When it comes to understanding chemical bonding and the intricacies of molecular structures, it’s always beneficial to explore additional resources. Here are some credible sources that delve deeper into the concepts of chemical bonding, Lewis dot diagrams, covalent bonds, and more:
- Chemical Bonding and Molecular Structure – This comprehensive textbook by P. Bahadur provides a thorough understanding of chemical bonding, valence electrons, and the octet rule. It also covers topics such as resonance structures, VSEPR theory, and molecular models. [^1^]
- Chemical Structure and Bonding – Written by Roger L. DeKock and Harry B. Gray, this book explores the fundamentals of chemical bonding, electron configuration, and molecular shape. It also delves into topics like atomic orbitals, bond angles, and the concept of hybridization. [^2^]
- Chemical Bonding: A Conceptual Approach – This textbook by G. Douglas and M. A. Morrison offers a conceptual approach to understanding chemical bonding. It covers topics such as Lewis symbols, chemical reactivity, and the relationship between atomic structure and chemical properties. [^3^]
- Chemical Bonding and Molecular Geometry – Authored by Ronald J. Gillespie and Paul L. A. Popelier, this book provides a comprehensive overview of chemical bonding and molecular geometry. It explores concepts like molecular shape, bond angles, and the distinction between polar and nonpolar molecules. [^4^]
- Chemical Bonding and Molecular Structure – This resource by K. K. Sharma offers a detailed explanation of chemical bonding, molecular structure, and the role of valence electrons. It also covers topics such as chemical compounds, structural formulas, and the notation used to represent chemical bonds. [^5^]
These sources will provide you with a solid foundation in understanding the principles of chemical bonding, Lewis dot diagrams, covalent bonds, and molecular geometry. Whether you’re a student or a curious learner, these references will help you explore the fascinating world of chemical structures and their properties.
[^1^]: Bahadur, P. (2006). Chemical Bonding and Molecular Structure. S. Chand Publishing.
[^2^]: DeKock, R. L., & Gray, H. B. (1989). Chemical Structure and Bonding. University Science Books.
[^3^]: Douglas, G., & Morrison, M. A. (1998). Chemical Bonding: A Conceptual Approach. Wiley.
[^4^]: Gillespie, R. J., & Popelier, P. L. A. (2001). Chemical Bonding and Molecular Geometry. Oxford University Press.
[^5^]: Sharma, K. K. (2009). Chemical Bonding and Molecular Structure. Krishna Prakashan Media.
Frequently Asked Questions
1. What is the Lewis structure for ClF?
The Lewis structure for ClF (Chlorine Monofluoride) involves a single covalent bond between the Chlorine and Fluorine atoms. Both atoms fulfill the octet rule, with Chlorine contributing 7 valence electrons and Fluorine contributing 7 as well. The remaining electron on Chlorine forms a lone pair.
2. How does the Lewis structure work?
Lewis structures are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. They help in understanding the arrangement of electrons in molecules, which is crucial for predicting chemical reactivity and properties of the compound.
3. Is ClF covalent or ionic?
ClF is a covalent compound. This is because both Chlorine and Fluorine are non-metals and they share electrons to achieve a stable electron configuration, forming a covalent bond.
4. How can you tell if a Lewis structure is polar or nonpolar?
A Lewis structure is polar if there is an asymmetry in the molecule’s structure, causing a distribution of charge. This can be determined by looking at the electronegativity of the atoms and the molecular geometry. If there is a difference in electronegativity and the molecule is not symmetrical, it is likely polar.
5. What is the Lewis structure for ClF3?
The Lewis structure for ClF3 (Chlorine Trifluoride) involves three bonds between the Chlorine and Fluorine atoms, with two lone pairs on the Chlorine atom. This results in a T-shaped molecular geometry due to the VSEPR theory.
6. Why is ClF3 T-shaped?
ClF3 is T-shaped due to the presence of two lone pairs of electrons on the Chlorine atom. According to the VSEPR theory, these lone pairs repel the bonded electron pairs, causing them to arrange in a way that minimizes repulsion, resulting in a T-shaped molecular geometry.
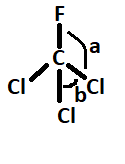
7. What is the Lewis structure for CHCl3?
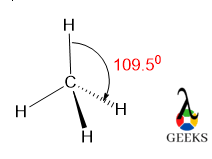
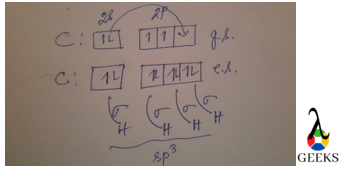
The Lewis structure for CHCl3 (Chloroform) involves a central Carbon atom bonded to a Hydrogen atom and three Chlorine atoms. The Carbon atom shares its four valence electrons with the other atoms to fulfill the octet rule.
8. Does CHCl3 have a coordinate bond?
No, CHCl3 does not have a coordinate bond. It has covalent bonds, where each atom shares electrons to fulfill the octet rule.
9. What is the Lewis structure for CH3Cl?
The Lewis structure for CH3Cl (Chloromethane) involves a central Carbon atom bonded to three Hydrogen atoms and one Chlorine atom. The Carbon atom shares its four valence electrons with the other atoms to fulfill the octet rule.
10. Where is CS Lewis buried?
C.S. Lewis is buried in the churchyard of Holy Trinity Church, Headington, Oxford, England.
Also Read: