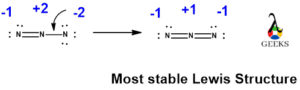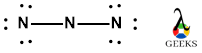The HF Lewis structure refers to the arrangement of atoms and electrons in a molecule of hydrogen fluoride. In this structure, the hydrogen atom (H) is bonded to the fluorine atom (F) through a single covalent bond. The Lewis structure helps us understand the bonding and electron distribution in a molecule. It is represented by drawing the atoms and their valence electrons, as well as the bonds between them. The HF molecule follows the octet rule, where each atom aims to have a full outer shell of electrons. The Lewis structure of HF is crucial in understanding the chemical properties and behavior of this compound.
Key Takeaways
The following table provides some helpful factual information about the HF Lewis structure:
| Atom | Valence Electrons |
|---|---|
| Hydrogen (H) | 1 |
| Fluorine (F) | 7 |
Please note that the table above is a concise summary and does not include any additional information or details.
Understanding the Basics of HF Lewis Structure
What is HF?
In chemistry, HF refers to hydrogen fluoride, which is a chemical compound composed of hydrogen and fluorine atoms. It is a colorless gas or a fuming liquid that is highly corrosive and toxic. HF is widely used in various industries, including the production of aluminum, petroleum refining, and the manufacturing of certain chemicals.
When it comes to understanding the Lewis structure of HF, we need to consider the arrangement of its valence electrons. Valence electrons are the electrons present in the outermost shell of an atom and play a crucial role in determining the chemical properties of an element.
HF Valence Electrons
Hydrogen (H) has one valence electron, while fluorine (F) has seven valence electrons. To determine the total number of valence electrons in HF, we add the valence electrons of hydrogen and fluorine together. Therefore, HF has a total of eight valence electrons.
The Lewis dot structure is a diagram that represents the valence electrons of an atom or molecule using dots. In the case of HF, we can represent the valence electrons of hydrogen and fluorine using dots around their respective atomic symbols.
HF Hybridization
Hybridization is a concept used to explain the bonding and molecular geometry of a molecule. In the case of HF, the hydrogen atom and the fluorine atom undergo hybridization to form a covalent bond.
Hybridization involves the mixing of atomic orbitals to form new hybrid orbitals. In HF, the hydrogen atom’s 1s orbital and the fluorine atom’s 2p orbital combine to form two new sp hybrid orbitals. These hybrid orbitals then overlap to form a sigma bond between the hydrogen and fluorine atoms.
The molecular shape of HF is linear, with a bond angle of 180 degrees. The presence of lone pairs on the fluorine atom affects the molecular geometry and gives rise to a bent molecular shape.
Understanding the basics of HF Lewis structure is essential for comprehending its chemical bonding, molecular structure, and properties. The Lewis dot structure, valence electrons, hybridization, and molecular shape are all interconnected concepts that contribute to our understanding of HF and other chemical compounds.
By applying principles such as the octet rule, VSEPR theory, and resonance structures, we can further analyze the electron pair distribution, bond angles, formal charge, and polarity of HF. These concepts help us visualize and predict the behavior of molecules in chemical reactions.
Steps to Draw HF Lewis Structure

Step 1: Determine the Number of Valence Electrons
To draw the Lewis structure of HF (hydrogen fluoride), we first need to determine the number of valence electrons present in the molecule. Valence electrons are the electrons in the outermost energy level of an atom and are crucial in determining the chemical properties of an element. In the case of HF, hydrogen (H) has 1 valence electron, and fluorine (F) has 7 valence electrons. Therefore, the total number of valence electrons in HF is 1 + 7 = 8.
Step 2: Identify the Central Element
The next step is to identify the central element in the molecule. In HF, hydrogen (H) is the only other element besides fluorine (F). Since hydrogen can only form one bond, it will always be the terminal atom in a Lewis structure. Therefore, fluorine (F) will be the central element in HF.
Step 3: Check the Octet Rule
The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with 8 electrons in their outermost energy level. In the case of HF, fluorine (F) needs only one more electron to complete its octet, while hydrogen (H) only needs two electrons. Since hydrogen can only form one bond, it will share one electron with fluorine. This shared pair of electrons is known as a covalent bond.
Step 4: Check Formal Charge
Formal charge is a way to determine the distribution of electrons in a molecule and helps us identify the most stable Lewis structure. To calculate the formal charge, we need to assign electrons to each atom in the molecule. In HF, hydrogen (H) shares one electron with fluorine (F), so hydrogen has a formal charge of 0, while fluorine has a formal charge of -1.
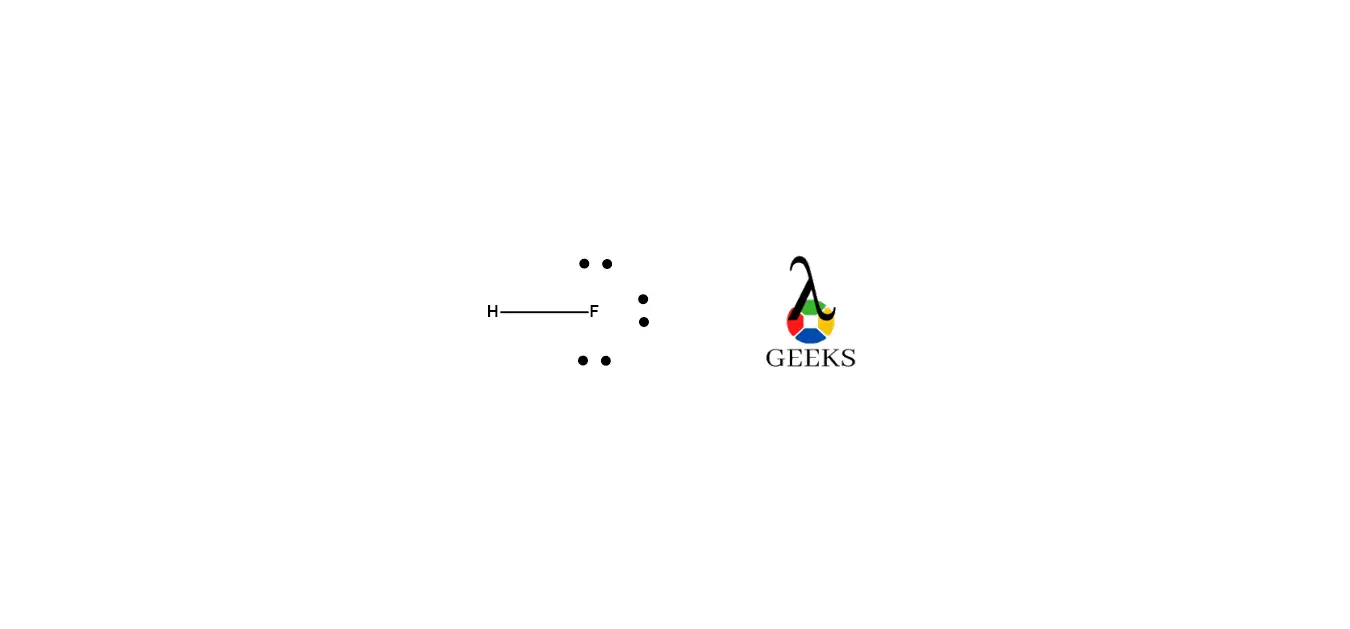
By following these steps, we can draw the Lewis structure of HF. The structure will consist of a single bond between hydrogen (H) and fluorine (F), with fluorine having a lone pair of electrons. The Lewis dot structure of HF can be represented as:
H: . F
Remember that Lewis structures are a simplified representation of molecular geometry and chemical bonding. They help us understand the arrangement of atoms and electrons in a molecule, but they do not provide information about the actual molecular structure, resonance structures, or bond angles. For a more detailed understanding of molecular shape and structure, other theories like VSEPR theory and hybridization of atomic orbitals can be used.
Characteristics of HF Lewis Structure
The Lewis dot structure is a representation of the valence electrons in a molecule and is used to predict the molecular geometry and chemical bonding. In the case of HF (hydrogen fluoride), the Lewis structure helps us understand its characteristics and properties.
HF Lewis Structure Shape
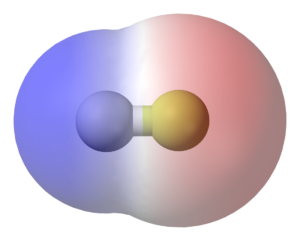
The shape of the HF molecule is determined by the arrangement of its atoms and lone pairs. According to the VSEPR theory (Valence Shell Electron Pair Repulsion theory), the HF molecule has a linear shape. This means that the hydrogen atom and the fluorine atom are in a straight line, with the hydrogen atom in the center and the fluorine atom on one end.
HF Lewis Structure Angle
The bond angle in the HF molecule is 180 degrees. This is because the molecule has a linear shape, and the bond between the hydrogen atom and the fluorine atom is a single bond. The VSEPR theory predicts that the bond angle in a linear molecule is 180 degrees.
HF Lewis Structure Lone Pairs
In the HF molecule, there are no lone pairs of electrons. Both the hydrogen atom and the fluorine atom contribute their valence electrons to form a covalent bond. A lone pair refers to a pair of electrons that is not involved in bonding and is localized on a specific atom.
HF Lewis Structure Formal Charge
The formal charge of an atom in a molecule is a measure of the electron distribution around that atom. In the HF molecule, the hydrogen atom has a formal charge of 0, while the fluorine atom has a formal charge of -1. The formal charge is calculated by subtracting the number of lone pair electrons and half the number of bonding electrons from the number of valence electrons.
HF Lewis Structure Octet Rule
The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with eight valence electrons. In the case of HF, both the hydrogen atom and the fluorine atom follow the octet rule. The hydrogen atom shares its electron with the fluorine atom, forming a covalent bond, and both atoms achieve a stable electron configuration.
Advanced Concepts Related to HF Lewis Structure
The HF Lewis structure is a representation of the chemical bonding in the HF molecule using Lewis dot structures. It provides valuable insights into the arrangement of atoms and electrons in a molecule. In this section, we will explore some advanced concepts related to the HF Lewis structure, including molecular geometry, electron geometry, polarity, and intermolecular forces.
HF Lewis Structure Molecular Geometry
The molecular geometry of a molecule refers to the three-dimensional arrangement of atoms in a molecule. In the case of HF, the central atom is hydrogen (H), and the surrounding atom is fluorine (F). The HF molecule has a linear molecular geometry, meaning that the hydrogen and fluorine atoms are in a straight line. This arrangement is due to the presence of only two atoms and no lone pairs of electrons around the central atom.
HF Lewis Structure Electron Geometry
The electron geometry of a molecule describes the arrangement of electron pairs around the central atom, including both bonding and lone pairs. In the case of HF, the electron geometry is also linear, as there are only two electron pairs around the central atom. The electron pair geometry is determined by the VSEPR (Valence Shell Electron Pair Repulsion) theory, which states that electron pairs repel each other and tend to be as far apart as possible.
HF Lewis Structure Polarity
Polarity refers to the separation of electric charge within a molecule. In the HF molecule, the fluorine atom is more electronegative than the hydrogen atom, resulting in a polar covalent bond. This means that the electron density is shifted towards the fluorine atom, creating a partial negative charge (δ-) on fluorine and a partial positive charge (δ+) on hydrogen. The polarity of the HF molecule gives rise to its unique properties and behavior in chemical reactions.
HF Lewis Structure Intermolecular Forces
Intermolecular forces are the attractive forces between molecules. In the case of HF, the polar nature of the molecule leads to the formation of dipole-dipole interactions. These interactions occur between the partially positive hydrogen atom of one HF molecule and the partially negative fluorine atom of another HF molecule. Dipole-dipole interactions are relatively strong intermolecular forces and contribute to the higher boiling point and melting point of HF compared to nonpolar molecules.
In addition to dipole-dipole interactions, HF molecules can also form hydrogen bonds. Hydrogen bonding occurs when a hydrogen atom is bonded to a highly electronegative atom (in this case, fluorine) and is attracted to another electronegative atom (such as oxygen or nitrogen) in a different molecule. Hydrogen bonding is a strong intermolecular force and plays a crucial role in various biological and chemical processes.
Understanding the advanced concepts related to the HF Lewis structure, such as molecular geometry, electron geometry, polarity, and intermolecular forces, provides valuable insights into the behavior and properties of HF and other chemical compounds. By analyzing the arrangement of atoms and electrons, we can gain a deeper understanding of the molecular structure and its impact on chemical reactions and properties.
HF Lewis Structure and Chemical Properties
Hydrogen fluoride (HF) is a chemical compound that consists of a hydrogen atom bonded to a fluorine atom. It is a covalent compound and is commonly used in various industrial applications. Let’s explore some of the key chemical properties of HF.
HF Solubility
HF is highly soluble in water. When HF is dissolved in water, it forms a solution known as hydrofluoric acid. This acid is corrosive and can cause severe burns. The solubility of HF in water is due to the formation of hydrogen bonds between the HF molecules and water molecules.
Is HF a Strong Electrolyte?
HF is a weak electrolyte. In aqueous solutions, HF partially dissociates into ions, producing a small concentration of hydrogen ions (H+) and fluoride ions (F-). However, the degree of dissociation is relatively low compared to strong electrolytes like hydrochloric acid (HCl).
Is HF a Hydrogen Bond?
Yes, HF can form hydrogen bonds. Hydrogen bonding occurs when a hydrogen atom is bonded to a highly electronegative atom, such as fluorine. In HF, the hydrogen atom is attracted to the lone pair of electrons on the fluorine atom, resulting in a strong dipole-dipole interaction.
Is HF Acidic or Basic?
HF is an acidic compound. When dissolved in water, it donates a proton (H+) to water molecules, resulting in the formation of hydronium ions (H3O+). This acidity is due to the partial dissociation of HF and the presence of hydrogen ions in the solution.
Is HF a Weak Acid?
Yes, HF is considered a weak acid. It does not completely dissociate into ions in water. Instead, only a small fraction of HF molecules dissociate, resulting in a relatively low concentration of hydrogen ions in the solution.
Is HF Stronger than HCl?
No, HF is not stronger than hydrochloric acid (HCl). HCl is a strong acid that completely dissociates into hydrogen ions and chloride ions in water. In contrast, HF is a weak acid with a lower degree of dissociation.
Is HF Polar or Nonpolar?
HF is a polar molecule. The fluorine atom is highly electronegative, causing it to attract the shared electrons in the HF molecule more strongly. As a result, there is an uneven distribution of electron density, with fluorine having a partial negative charge and hydrogen having a partial positive charge.
Is HF a Lewis Acid or Base?
HF can act as both a Lewis acid and a Lewis base. As a Lewis acid, it can accept an electron pair from a Lewis base. On the other hand, as a Lewis base, it can donate an electron pair to a Lewis acid. The ability of HF to act as both an acid and a base is due to the presence of a lone pair of electrons on the fluorine atom.
Is HF Linear?
HF, which stands for hydrogen fluoride, is a molecule composed of one hydrogen atom and one fluorine atom. When determining the molecular geometry of a molecule, we consider the Lewis dot structure, valence electrons, and the concept of chemical bonding. In the case of HF, the Lewis dot structure shows that hydrogen contributes one valence electron, while fluorine contributes seven. This gives us a total of eight valence electrons.
To understand the molecular geometry of HF, we need to consider the electron pair arrangement and the molecular structure. According to the octet rule, atoms tend to gain, lose, or share electrons to achieve a stable electron configuration. In the case of HF, hydrogen and fluorine share electrons through covalent bonding, resulting in a molecule with a linear molecular structure.
The VSEPR (Valence Shell Electron Pair Repulsion) theory helps us determine the molecular shape of HF. In this theory, electron pairs around the central atom repel each other and arrange themselves to minimize repulsion. Since HF has two electron pairs (one bonding pair and one lone pair), the molecular shape is linear.
Now, let’s move on to the question of whether HF is paramagnetic or diamagnetic.
Is HF Paramagnetic or Diamagnetic?
To determine if a molecule is paramagnetic or diamagnetic, we need to consider its electron configuration and the presence of unpaired electrons. Paramagnetic molecules have unpaired electrons, while diamagnetic molecules have all their electrons paired.
In the case of HF, the fluorine atom has seven valence electrons, and the hydrogen atom contributes one. When these electrons combine, they form a covalent bond, resulting in a molecule with a total of eight electrons. Since all the electrons in HF are paired, it is considered diamagnetic.
It’s important to note that the molecular structure and electron configuration play a crucial role in determining the magnetic properties of a molecule. By understanding the Lewis dot structure, valence electrons, and molecular geometry, we can determine whether a molecule is linear and whether it is paramagnetic or diamagnetic.
To summarize, HF has a linear molecular structure due to the sharing of electrons between hydrogen and fluorine atoms. It is considered diamagnetic since all its electrons are paired. These concepts of molecular shape and magnetic properties are fundamental in understanding the behavior of chemical compounds and their involvement in various chemical reactions.
References
In the study of chemistry, understanding the structure and properties of molecules is crucial. Several concepts and theories help us comprehend the intricacies of molecular structure, such as Lewis dot structures, valence electrons, and molecular geometry. These concepts play a significant role in explaining chemical bonding and the overall behavior of molecules.
One fundamental concept is the Lewis dot structure, which represents the arrangement of valence electrons in a molecule. Valence electrons are the outermost electrons involved in chemical bonding. By using Lewis dot structures, we can determine the number of valence electrons and predict the molecular geometry.
The VSEPR (Valence Shell Electron Pair Repulsion) theory is another important concept that helps us understand molecular geometry. According to this theory, electron pairs in the valence shell of an atom repel each other, resulting in specific bond angles and molecular shapes. The octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight valence electrons, is also closely related to molecular geometry.
Chemical bonding occurs when atoms share or transfer electrons to achieve a stable electron configuration. Covalent bonding, where atoms share electron pairs, is a common type of chemical bonding. The concept of hybridization explains how atomic orbitals combine to form hybrid orbitals, which in turn determine the molecular structure and shape.
Resonance structures are alternative Lewis dot structures that represent the delocalization of electrons within a molecule. They help us understand the stability and reactivity of chemical compounds. Polarity is another important aspect of molecular structure, which depends on the distribution of electron density within a molecule. It is determined by factors such as electronegativity difference and molecular shape.
To visualize and study molecular structures, various molecular models are used. These models provide a three-dimensional representation of molecules, allowing us to analyze bond angles, lone pairs, and overall molecular shape. Molecular models help us understand the spatial arrangement of atoms and predict the behavior of molecules in chemical reactions.
Frequently Asked Questions
Q1: What is the Lewis structure in chemistry?
A: The Lewis structure in chemistry is a graphical representation of the atomic structure and electron configuration of a molecule. It shows how the valence electrons are arranged among the atoms in the molecule, which helps to predict the molecule’s shape, polarity, and reactivity.
Q2: Is HF a Lewis acid or base?
A: HF, or Hydrogen Fluoride, is considered a Lewis acid. This is because it can accept a pair of electrons during a chemical reaction, which is the defining characteristic of a Lewis acid.
Q3: Where are Lewis and Clark buried?
A: Meriwether Lewis is buried near Hohenwald, Tennessee, while William Clark is buried in Bellefontaine Cemetery, St. Louis, Missouri.
Q4: Where are Lewis and Clark from?
A: Meriwether Lewis was born in Albemarle County, Virginia, and William Clark was born in Caroline County, Virginia.
Q5: What is the HF Lewis structure?
A: The HF Lewis structure consists of a single bond between the Hydrogen and Fluoride atoms, with three lone pairs of electrons on the Fluoride atom. This structure satisfies the octet rule for Fluoride.
Q6: What is the molecular geometry of HF according to the VSEPR theory?
A: According to the VSEPR (Valence Shell Electron Pair Repulsion) theory, the molecular geometry of HF (Hydrogen Fluoride) is linear.
Q7: Does HF have resonance structures?
A: No, HF does not have resonance structures. Resonance structures occur when there are multiple valid ways to place the pi bonds and non-bonding lone pairs of electrons, but in HF there is only one single bond and no pi bonds.
Q8: Does HF form hydrogen bonds?
A: Yes, HF does form hydrogen bonds. The hydrogen in HF can form a bond with the lone pair of electrons on a Fluoride atom in another HF molecule, creating a strong intermolecular force.
Q9: What is the electron geometry of the HF Lewis structure?
A: The electron geometry of the HF Lewis structure is tetrahedral. This is because there are four regions of electron density around the Fluoride atom – one from the bond with Hydrogen and three from the lone pairs of electrons.
Q10: Is HF a linear molecule?
A: Yes, HF is a linear molecule. Despite its tetrahedral electron geometry, the molecular geometry (shape) of HF is linear because there is only one bond and three lone pairs on the Fluoride atom.
Also Read: