In this article, we discuss what is peptide bond, peptide bond structure, synthesis, and detailed facts.
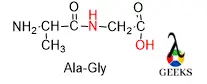
Before starting with a peptide bond, we first know a peptide bond is nothing but a combination of two or more amino acids. The N terminal of one amino acid gets attached to the C terminal of another amino acid and formed a peptide bond. This peptide bond can form a protein structure.
If amino acid contains any aromatic group then they can form a tertiary protein structure. In short peptide bonds are nothing but a polymer of amino acid linked with amino acids via amide bond with loss of water.
Peptide bond formula
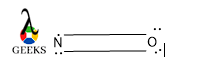
If we consider a peptide bond structure then we can easily find out the peptide bond formula. The formula of the peptide bond is R1-CONH-R2. Where -CONH- is the amide bond linkage and R1 and R2 are the side chain of two different amino acids.
Peptide bond structure
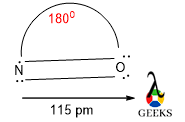
Peptide bond structure is rigid, planner, and trans. It shows a partial double bond character due to the resonance effect between N of amide and O of the carboxyl group.
Here hydrogen from the amide group and O from the carboxyl group lie trans to each other.
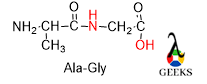
Synthesis of Peptide bond
There are five-step to syntheses a peptide bond, they are listed below
- N-protection of N-terminal Amino acid
- C-protection of C-terminal Amino acid
- Activation of -COOH group of N-protected N-terminal Amino acid
- Amide linkage formation
- De-protection
N-protection of N-terminal Amino acid (Alanine) using tboc functionality
In peptide bond structure the lone pair over N is attacked on the carbonyl carbon of tboc functionality and get protected Amine group, so it cannot further react with another reagent.

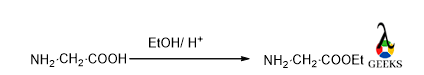
C-protection of C-terminal Amino acid (Glycine) by ethanol in presence of acid
In presence of strong acid and ethanol, the acid group is converted into ester, it is a simple esterification reaction. So, this carboxyl group get protected or locked and did not interfare any further reaction.
Activation of -COOH group of N-protected N-terminal Amino acid
As Carboxylic acid is less reactive due to the presence of the carboxyl group, so it needed to be activated to participate in the desired reaction.
So, we need an activating agent which can activate the carboxylic group.
We use here di-cyclohexyl carbodiimide for activating the carboxylic group by converting it into an amide. Amide has greater reactivity than the carboxylic group.
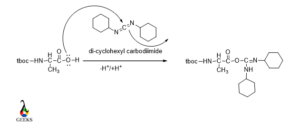
The lone pair over O in the carboxylic group attacked the carbon center in DCC and the carboxylic group converted in the amide group.
Amide linkage formation /Peptide bond formation
Now it’s time to make a peptide bond via loss of water between N-protected amino acids and C-protected amino acids.
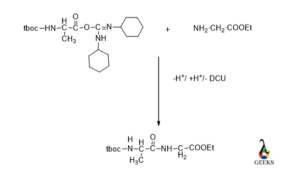
De-protection
Now its time to de protected the N terminal and C terminal of amino acids to get original peptide bond.
Tboc functionality can be removed by mild basic condition or using TFA/CH2Cl2 and ester part removed by basic condition.
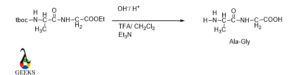
The name of the peptide bond is according to the first 3 letters of each amino acid and the first name starts with that amino acid whose N terminal gets protected.
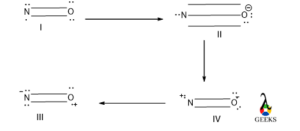
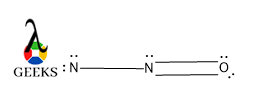
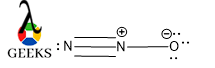
Peptide bond resonance structure
Yes, there is a possible resonating structure in a peptide bond structure. As the structure of peptide bond is a planner so all the molecules are supposed to be lies in the same plane and resonance occurs within the amide group in between C=O and N atoms which are attached with that C.

Is peptide bond structure formed during transcription?
In peptide bond structure a transcription factor recognizes the certain region of DNA that controls the genetic code in RNA. DNA protein can form by ZN-fingers and these Zn-fingers contain Cysteine -S donor and histidine-N donor. At last, they form α -helix. Cysteine and histidine are amino acids and they can form peptide bonds in transcription.
The characteristics residue of Zn-fingers is
-(Tyr, Phe)-X-Cys-X2-4-Cys-X3-Phe-X5-Leu-X2-His-X3-5-His-
Where X is variable amino acids. Zn is particularly suitable for binding protein in a particular confirmation according to the Irving-William series and thus makes a stable complex via S and N donors. This is a redox inactive protein so it can avoid the oxidative damage of DNA.
Peptide disulfide bond structure
Many proteins, peptides, and enzymes evolved several defense mechanisms preventing them from denaturation or degradation. Di sulfide bond is one of the protective techniques. Disulfide bond increases the thermodynamic stability of a peptide as well as protein. A disulfide bond can save a peptide bond from high temperature, very acidic or basic pH, and a high concentration of organic solvents by increasing the half-life of the peptide.
Generally, disulfide bonds stabilize the properly folded proteins and destabilized the denaturant.
Mainly disulfide bond can see in those peptides formed from Cysteine amino acid. There are two mechanisms of forming disulfide bonds, one is the chemistry of thiol/sulfide exchange and another is the kinetics and thermodynamics of oxidative folding.
In the 1st step reactivity of Cysteine thiolate will be performed then mixed disulfide is broken by nucleophilic attack from 2nd protein thiolate. As thiol removed as leaving group by the cysteine thiolate.
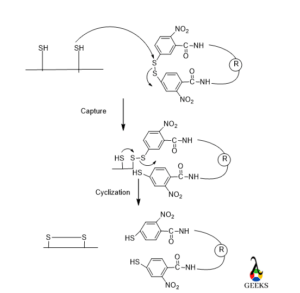
Peptide bond structure in protein
There are mainly four types of protein structures
- Primary – Assembly
- Secondary- folding
- Tertiary-packing
- Quarternary-interaction
Primary structure
The Assembly occurs at the ribosome for the primary structure. Primary structure involved in dehydration synthesis of proteins and polymerization of amino acids which are attached to tRNA:
NH3+ – {A + B à A-B + H2O}n -COO–
The above process is thermodynamically unfavorable as the change in energy i.e. DE = +10kJ/mol, so the change in Gibb’s free energy will be positive. The primary structure is linear, ordered, and 1 dimensional. It has a special sequence of amino acids which are in some order. By convention, the name of the primary structure is written from the N terminal end to the C terminal end.
For a primary structure, a perfectly linear amino acid polymer is useless as the function of linear amino acid is void and energetically unfavorable.
Secondary structure
In secondary Structure, protein gets folded. The process of folding occurs in the cytosol. The secondary structure of a protein is involved in spatial interaction among amino acids. The secondary structure may or may not involve chaperone proteins but the process is thermodynamically not the favorable value of change in energy is very low.
The structure of a secondary protein is non-linear and 3-dimensional. The stabilization factors of secondary protein are hydrogen bonding, electrostatic force, and van der Waal attraction.
Secondary structure determination
Random coil (Unfolded state)
positive at 212 nm (π->π*)
negative at 195 nm (n->π*)
b -Sheet
negative at 218 nm (π->π*)
positive at 196 nm (n->π*)
a-helix
positive (π->π*)perpendicular at 192 nm
negative (π->π*)parallel at 209 nm
negative at 222 nm is redshifted (n->π*)
Tertiary structure
Packing of a protein occurs in the cytosol (~60% bulk water, ~40% water of hydration). Chaperons and membrane proteins promoted the process where solvent and secondary structure of protein gets interacted. Tertiary structure tumbles into molten globule states. This is an essential part. The process is thermodynamically unfavorable as the overall entropy of this reaction decreases due to the hydrophobic effect. Then it is needed for the formation of, the tertiary structure.
The structure of a tertiary protein is non-linear and 3-dimensional like a secondary structure. The stabilization factor of the tertiary structure is hydrogen bonding, hydrophobic packing even sometime covalent bonds like disulfide bond formation. A globular amino acid polymer is folded and its function is catalytic and it is an energetically favorable Process.
Quarternary Structure
Interaction occurs in the cytosol, which is very close to other folded and arranged packing proteins so that interaction may be strong enough. The process of interaction in quarternary structure gets promoted by Chaperones, membrane proteins, and cytosolic and extracellular elements. The DE of the process decreases. Here desolvation occurs that results in a reduction of surface area.
Globular protein is an example of a quarternary structure, e.g. hemoglobin.
The quarternary structure is largely involved in the catalytic role. The quarternary structure is also fibrous proteins, e.g. collagen, that plays an important role in structural determination. This way quarternary structure is formed. The quarternary structure is non-linear, 3-dimensional. It is also involved in global, and across distinct amino acid polymers in different amino acid sequences. Hydrogen bonding, covalent bonding, hydrophobic packing, and hydrophilic exposure stabilized the quarternary structure.
FAQ
Why peptide bond is not involved in tertiary structure?
Actually, tertiary protein structure is formed due to the interaction of the R group of amino acids.
These alkyl group interactions may involve hydrogen bonding, ionic bonding, dipole-dipole interactions, London dispersion forces, van der Waal’s interaction, and some time may be disulfide bonds also. Also, there is sometime hydrophobic interaction that occurs in amino acids which are nonpolar. So, there is no chance of formation of amide linkage or peptide bond formation in tertiary structure.
Why Peptide bond is a partial double bond?
Due to resonance between C=O and C-N of the amide group, there is delocalization of electron and there will be a partial C=N bond formed. This only happens when amino acids form a peptide bond. So, the peptide bond contains a partial double bond.
Why peptide bond is planar?
In a peptide bond, all the carbon atoms of individual amino acids are sp2 hybridized.
So, they are planar and lying in the same plane, It is also evident that it is possible for resonance in peptide bond and resonance occurs only all the atoms are present in the same plane. So, the peptide bond is planar.
Read more about following Structure & Characteristics