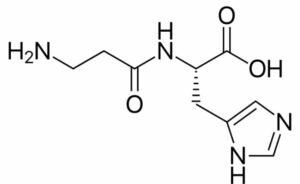
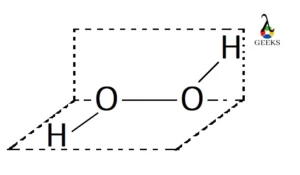
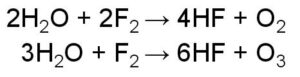
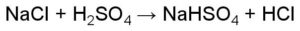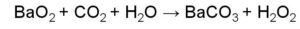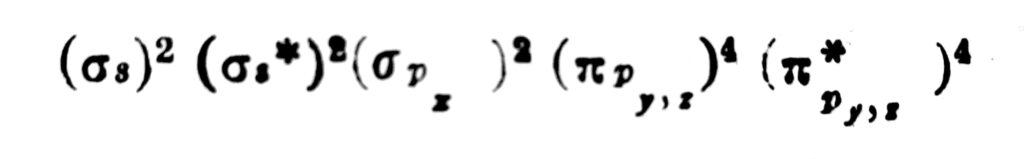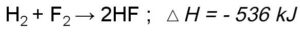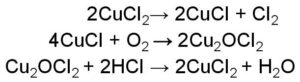Peptide bond example is the one wherein the linking of the atoms is through a peptide bond.
What we understand by a peptide bond is bond formation takes place when two amino acids combine together and form a bond, so basically, it is the linking of amino acids. Based on the number of amino acids coming together or combining peptide bonds can be classified as dipeptide bond, tripeptide, and so on.
Peptide bond examples:
Valine
Its synonym is 2-Amino-3-methylbutanoic acid. It was initially isolated in 1901 (Herman Emil Fischer) from Casein. It is an -amino acid that is used for synthesis of proteins (biosynthesis).
Coming to the chemical formula of valine it has six carbon atoms, eleven hydrogen atoms, one nitrogen atom, and two oxygen atoms. The molar mass is observed to be around 117.148g mol-1 and density is 1.316g/cm3. The melting point of valine is around 298 degrees Celsius and is almost soluble in water. It is a very important amino acid for the human body (our human body does not synthesize on its own) hence can be fulfilled by consuming food that are sources of valine.
Image credit : Wikipedia
Sources include meat, various dairy products, beans, etc. The significant functions of valine include required for metabolism of muscles, repairing of tissues and most important maintaining proper balance of nitrogen in human body.
Biosynthesis of Valine
Pyruvate undergoes acetohydroxy acid synthase and gives alpha-Acetolacetate.
Alpha-Acetolacetate undergoes acetohydroxy acid isomeroreductase and yields alpha, beta-dihydroxy-isovalerate.
Alpha, beta-dihydroxy-isovalerate undergoes Dihydroxy acid dehydratase and alpha-ketoisovalerate is obtained.
Alpha- ketoisovalerate undergoes aminotransferase giving Valine.
Glucagon
Its synonym is glucagonum and glucagone. It was discovered as the hyperglycemic factor (in pancreas) in the year 1922 by C.Kimball and John R. Murlin. Its chemical formula is C153H225N43O49Sand the molecular weight is 348.7.
In appearance, it is a white colored powder (crystalline) and does not have characteristic odor. Talking about its solubility, it is soluble in alkaline and acid type of solution but seems to be insoluble in organic solutions and water. It is stable for around almost 48 hours if stored at a temperature of 5 degrees Celsius. Being a peptide hormone it is secreted in the pancreas by the alpha cells.
It is considered as a catabolic hormone as it increases glucose, fatty acids concentration in the bloodstream. Coming to glucagon preparation it is obtained when proglucagon is cleaned in pancreatic islet alpha cells by proprotein convertase 2.
Read more about : Disulfide reduction: How, What, Methods and Several Facts
Carnosine
Its synonym is beta-Alanyl-L-histadine. It was isolated in 1900 from extract of muscle skeletal by Vladimir S.G. It is made up of beta-alanine, histadine (amino acids).
Taking into account the chemical formula, it has nine atoms of carbon, fourteen atoms of hydrogen, four atoms of nitrogen, and three atoms of oxygen. In appearance, it is almost solid (crystalline). It has a molar mass of 226.236g mol-1. It is observed to melt at a temperature of 253 degrees Celsius. Unlike valine, Carnosine can be secreted in the body (in the liver).
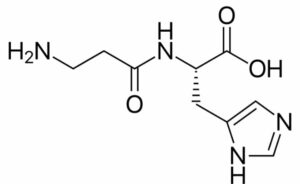
It has a pKa=6.83 and therefore acts as a good buffer (especially for animal muscles pH). It is used for many medicinal purposes like treatment related to diabetes, damage of nerves, and disorder of eyes, etc.
Opthalmic acid
Its synonym opthalmate (it is a tripeptide). This acid was isolated initially from lens of calf.
The chemical structure includes eleven atoms of carbon, nineteen atoms of hydrogen, nine atoms of nitrogen, and six atoms of oxygen. Its observed molar mass is around 289.288g mol-1, in appearance it is solid and colorless (crystals). It plays a role in the metabolism of humans also acts as an indicator for the various biological states.
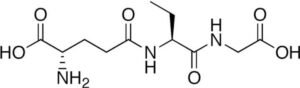
It finds important application in food and pharmaceutical industries. It can be synthesized (biosynthesis) through the biological method by carrying out reactions between 2-amino butyric acid and gamma-glutamylcysteine, glutathione synthetase.
Oxytocin
This peptide is made up of 9 amino acids. Its synonym is Pitocin. It was first discovered in the year 1909 by Henry H.D.
Its molecular formula includes forty-three atoms of carbon, sixty six atoms of hydrogen, twelve atoms of nitrogen, twelve atoms of oxygen, and two atoms of sulfur. Its molecular weight is 1007.2. In appearance, it is white in color (powder). Talking about the solubility, it is soluble in water as well as in alcohol like butanol. When oxytocin is heated it decomposes and gives out fumes of sulfur, nitric oxides which are toxic in nature.
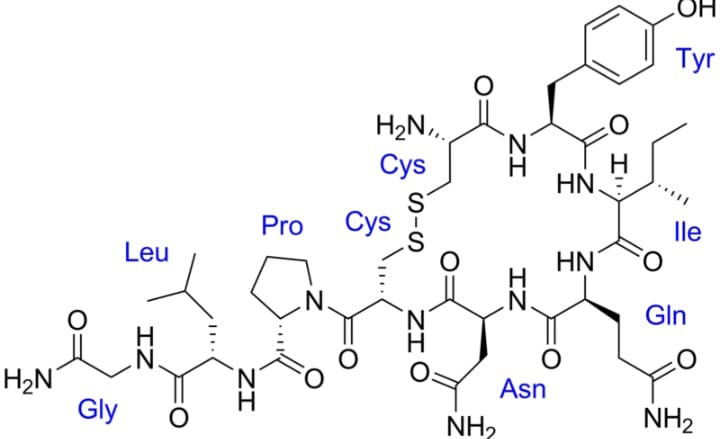
Oxytocin’s structure is almost similar to that of vasopressin. It is formed in hypothalamus (by posterior pituitary). It is released in the women’s body at the time of childbirth required for contraction of uterine during delivering the baby.
Read more about : Disulfide Bonds Examples : Several Facts
Alanine
Its synonym is 2-Aminopropanoic acid. It was first isolated in 1850 by Adolph S from natural substances. This amino acid is very essential for the process of biosynthesis of various proteins.
Its chemical formula includes three atoms of carbon, seven atoms of hydrogen, one atom of nitrogen, and two atoms of oxygen. Its observed molar mass is 89.094g mol-1 and in appearance, it is a white-colored powder. It is said to have a density=1.424g/cm3 and melts at a temperature of 258 degrees Celsius. It is soluble in water (at a temperature of 25 degrees Celsius). The free radical (CH3C.HCO–2) is produced on carrying out deamination of molecule of alanine.
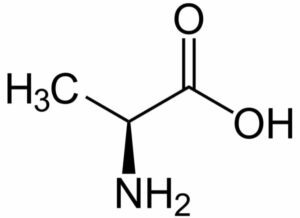
This property finds its application in radiotherapy (for dosimetric measurement). In living beings, the amino acid alanine has a significant role in the cycle of glucose-alanine (between the tissues and liver). Talking about the synthesis, it can be prepared by biosynthesis by the reaction of pyruvate with valine, leucine, and isoleucine (preferably amino acids having branched chain). There is one more method (chemical synthesis) for preparing alanine by carrying out condensation of acetaldehyde and ammonium chloride (sodium cyanide should be necessarily present in the reaction mixture.
Glycine
Its synonym is glycol. It was initially isolated by Henri B. in the year 1820. Glycine is one of the simplest amino acid. Its chemical formula includes two atoms of carbon, five atoms of hydrogen, one atom of nitrogen, and two atoms of oxygen.
Its observed molar mass is around 75.067g mol-1 and in appearance, it is a white-colored solid. Its density is 1.1607g/cm3 and melts at a temperature of 233 degrees Celsius. It is found to be soluble in water (at a temperature of 25 degrees Celsius), pyridine. Glycine finds its application in the synthesis of various kinds of chemicals (acts as an intermediate) such as imiprothrin, iprodione, herbicides glyphosphate. Most important, it is used for preparing thiamphenicol a very essential medicine.
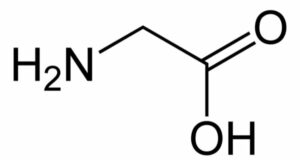
Also acts as a buffer during the process of electrophoresis for maintaining the pH and to prevent the sample from getting damaged. Many foods are sources of glycine such as peanuts etc. Talking about its chemical properties, it has both acid as well as base. Glycine is amphoteric in aqueous solution having a pH lower than 2.4, it is seen to convert into ammonium cation and above the pH=9.4 it converts into glycinate. We can prepare glycine by carrying out amination on chloroacetic acid and ammonia.
Glutathione
Its synonym is gamma-L-Glutamyl-L-cysteinylglycine. It was first isolated by Frederick G. Hopkins in the year 1929 from yeast. Its chemical formula has ten atoms of carbon, seventeen atoms of hydrogen, three atoms of Nitrogen, six atoms of oxygen, and one atom of sulfur.
Its observed molar mass is 307.32g mol-1 and melts at a temperature of 195 degrees Celsius. It is soluble in water and insoluble in alcohol like methanol and ethers. Glutathione acts as an antioxidant by protecting the cells from reactive species such as oxygen by neutralizing. Talking about its applications, it is used for making wine. Also used in the cosmetic industry. Glutathione can be prepared by the biosynthesis process involving two steps.
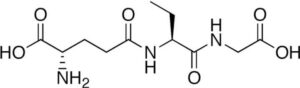
In the first step, L-glutamate is reacted with cysteine to give gamma-glutamylcysteine, upon adding glycine to the obtained product at C-terminal yield our required peptide. Human beings can synthesize Glutathione in the body. Let’s have a look at methods of determining Glutathione. One method involves extracting thiols by using a buffer (HCL) and then reducing the thiols by dithiothreitol and further labelling by monobromobimane. The monobromobimane once binds to glutathione becomes fluorescent and at later stages, the thiols can be separated (using the HPLC method).
Calcitonin
Its synonym is thyrocalcitonin. It was discovered by Douglas H.C, B. Cheney in the year 1962.
It is a hormone (peptide) consisting of around 32 aminoacids. There is disulfide linking/bridging between residues of cysteine at 1, 7 positions also there is prolinamide at the terminus (carbonyl). It is released by parafollicular cells (of the thyroid). Its important function includes seen to cause reduction of calcium in the blood thus opposing parathyroid hormone effects and also plays a role in the metabolism of phosphorus and calcium.

Most important application is in recognizing nodular thyroid disease patients. Also used in treating diseases such as osteoporosis.
Amanitin
Its synonym is alpha-amanitine. It was isolated somewhere around 1900.
Its molecular formula has thirty nine atoms of carbon, fifty four atoms of hydrogen, ten atoms of nitrogen, fourteen atoms of oxygen, and one atom of sulfur. Its observed molar mass is around 918.97g mol-1 and melts at a temperature around 255 degrees Celsius. It is soluble in water. This particular compound is observed to be very hazardous.
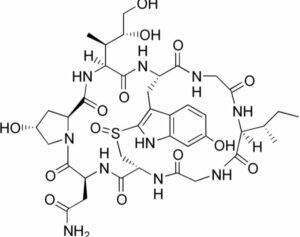
It is quite strong, specific, and can bind to enzyme RNA polymerase (II) and leads to cytolysin (of hepatocytes in liver cells). And this gives rise to symptoms like cramps and diarrhea eventually leads to failure of kidneys and liver. At times can be fatal as well. Hence while handling it one should be very careful.
Anserine
Its synonym is beta-Alanyl-3-methyl-L-histidine.
Its chemical structure has ten atoms of carbon, sixteen atoms of hydrogen, four atoms of nitrogen, and three atoms of oxygen. Its observed molecular weight is 240.26. In appearance, it is solid. Its melting point is around 227 degrees Celsius and boils at a temperature of 611.30 degrees Celsius. As we know it is a dipeptide (consisting of beta-alanine, 3-methyl-L-histidine units).
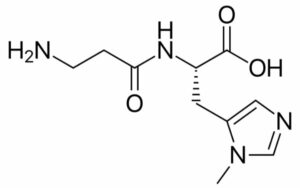
Mostly red meat is the source of anserine. It can also act as an antioxidant.
Also Read: