In this article we will discuss about the H2CO Lewis structure , characteristics : 41 complete facts
Formaldehyde (H2CO) is a gaseous organic compound, which has an irritating and pungent smell. It is present in its aqueous form which is white in color.
How to draw H2CO Lewis structure ?
In the H2CO Lewis structure, Hydrogen (H)and oxygen (O) is a gas and carbon (C) is metal, So, the hydrogen atom in the formaldehyde (H2CO) has 1 valence electron but the formaldehyde molecule is formed by carbon, oxygen and two hydrogen atoms. In the H2CO molecule, the carbon atom has 4 valence electrons, hydrogen has 1 valence electron and the oxygen atom has 6 valence electrons.
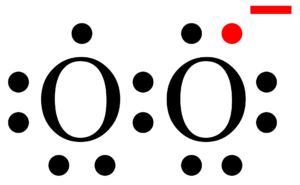
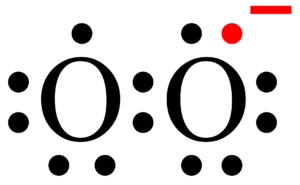
Hence in total, H2CO have 2+4+6 = 12 valence electrons. out of the three atoms, carbon is the least electronegative atom (2.55), so while drawing Lewis’s structure of H2CO we can put carbon (C) in the center and two hydrogen atoms and one oxygen (O) atom placed around it. Then connect the carbon (C) and two hydrogen atoms with single bonds while one oxygen forms a double bond with central carbon (C) We’ve used 12 electrons so far (one bond = two electrons).
Therefore, carbon shares its six valence electrons with two hydrogen atoms and one oxygen atom. In comparison, the oxygen atom has four valence electrons so for completing their octet it forms a double bond (1 double bond = 4 electrons) with carbon and has two lone pair on itself and fulfills their octet. Two hydrogens used remaining two electrons for completing their octet.

H2CO Lewis structure resonance
Due to the symmetrical structure of the H2CO molecule, it shows resonating structures also this molecule contains a double bond and lone pairs for delocalization of electrons. because the resonating structure of molecules is formed in the symmetrical structure with a double bond or lone pair for the delocalization of electrons.
Hence H2CO molecule shows resonating structure by delocalization of double bond which is formed in between carbon and oxygen. And also, lone pair of oxygen atoms undergo electrons rearrangement and form different resonating structures.
H2CO lewis structure shape
Formaldehyde molecule (H2CO) shows trigonal planer geometry because it has AX3 type molecule which has carbon atom in the centre which is surrounded by two hydrogens and one oxygen atom with two lone pairs.
In the trigonal planer geometry of formaldehyde (H2CO) central atom is carbon which is attached to two hydrogen atoms by a single C-H bond and oxygen is attached with a double bond.

H2CO Lewis structure formal charge
The formal charge of all the atoms in the formaldehyde (H2CO) molecule is zero. We calculated the formal charge of formaldehyde (H2CO) by using the below formula, The Lewis structure formal charges of H2CO can be calculated by the following formula FC = V – N – B/2 Where V = no. of valence electrons, N = no. of non–bonding electrons, B = no. of bonding electrons
The formal charge of the formaldehyde (H2CO) Lewis’s structure
FC of C in H2CO Lewis structure = 4 – 0 – 8/2 = 0 , FC of H in H2CO Lewis structure = 1-0-1/2 = 0 , FC of O in H2CO molecule = (6 – 4 – 4/2) = o
H2CO Lewis structure angle
The Formaldehyde (H2CO) is trigonal planer geometry with a bond angle of 120o.
H2CO Lewis structure octet rule
In the Formaldehyde (H2CO) molecule carbon (C) requires four electrons in its outermost orbital to complete its octet. A hydrogen atom has one valence electron it can donate its one electron to a carbon atom and form a C-H bond between them this way carbon, also oxygen require two electrons for completing their octet. hydrogen and oxygen atoms and carbon atoms share electrons and complete their octet and become stable.
H2CO Lewis structure lone pairs
In formaldehyde (H2CO) carbon atoms do not contain lone pair of electrons Also, hydrogen atoms do not contain lone pairs but the oxygen atom has two lone pairs of electrons.
H2CO valence electrons
In the H2CO molecule valence electrons are present in the Carbon atom = 4, Valence electrons are present in two hydrogen atoms = 2, and Valence electrons are present in the oxygen atom = 6, Therefore, in the H2CO molecule total number of valence electrons is 12 (4+2+6=12).
Hence in total, H2CO have 2+4+6 = 12 valence electrons. out of the three atoms, carbon is the least electronegative atom (2.55), so while drawing Lewis’s structure of H2CO we can put carbon (C) in the center and two hydrogen atoms and one oxygen (O) atom placed around it.
Read more about Hexanol Structure and Characteristics
H2CO hybridization
The formaldehyde (H2CO) molecule shows Sp3 hybridization the hybridization is decided according to the steric number of that molecule. The steric number of any molecule is calculated by adding bonding pair of electrons, a nonbonding pair of electrons and lone pair of electrons.
H2CO solubility
Formaldehyde is polar in nature hence it dissolves in polar solvents like water (H2O) and ammonia (NH3) and is soluble in non-polar solvents. Because polar molecule has the ability to dissolve in polar solvent only and non-polar molecule dissolves in non-polar solvent only. Yes H2CO is soluble in water and other polar solvents also like NH3
Is H2CO an electrolyte ?
The molecule is said to be a strong electrolyte when it is in solution and conducts electricity. While molecule is said to be nonelectrolyte when it does not conduct electricity. H2CO solution does not conduct electricity hence it is non-electrolyte.
Is H2CO acidic or basic ?
H2CO molecule is week acidic in nature . Because the formaldehyde molecule is able to donate only one proton or H+ ion. Formaldehyde does not act as a Arrhenius acid because it donate only one proton.
Is H2CO an Arrhenius acid ?
No formaldehyde (H2CO) is not an Arrhenius acid , because when it dissolve in water it can not dissociates into H+ ion .
Is H2CO polyprotic acid ?
No, H2CO (formaldehyde) is not a poly protic acid because it donated only one proton or H+ ion to another chemical molecule . It donate only one proton or H+ ion when dissolve in water hence it is monoprotic acid not polyprotic acid.
Is H2CO polar or nonpolar ?
Formaldehyde (H2CO) is formed by combining one carbon, two hydrogens and one oxygen atom the electronegativity of the carbon atom is 2.55 and that of electronegativity of the hydrogen atom is 2.20 and that of oxygen is 3.44 the difference between the electronegativity of hydrogen and carbon is 0.35. while that of electronegativity of carbon and oxygen is 0.89. Due to very low electronegativity difference H2CO is polar in nature.
Is H2CO linear ?
H2CO molecule is not linear it is a trigonal planer geometry. Because it shows AX3 hybridization.
Is H2CO paramagnetic or diamagnetic ?
Formaldehyde (H2CO) shows paramagnetic nature because all atoms in the H2CO (Formaldehyde) contains unpaired hence it is weekly attracted by the applied magnetic field and forms an induced magnetic field in the opposite direction Also, those atoms, ions or molecule which contain lone pair electrons or vacant outermost orbital has a paramagnetic nature while that atom, ion or molecule containing all paired electrons are diamagnetic.
H2CO boiling point
The Boiling point of H2CO is -190C.
H2CO bond angle
The bond angle in formaldehyde (H2CO) is close to 120o, the bond angel between H-C-H is < 120o and O-C-H is > 120o
Is H2CO diprotic?
Diprotic acids are the acids which they contain two hydrogens atoms in a molecule which are capable of dissociating in water means they can donate one proton while the dissociation process. Therefore, H2CO is diprotic because this molecule contains two hydrogen atoms which donate one proton while the dissociation process.
Is H2CO ionic or covalent?
Ionic bonds are the chemical bonds which are formed by the attraction between opposite charge atoms, covalent bonds are the chemical bond which is formed by the sharing of electrons. In the case of the H2CO molecule, the chemical bonds are formed by the sharing of electrons between hydrogen, carbon and oxygen hence Formaldehyde (H2CO) is a covalent compound.
Is H2CO amphiprotic?
When the molecule is said to be amphoteric when it acts as both acidic and basic, means it plays an important role in donating electrons as well as in accepting electrons. H2CO molecule does not act as both acidic and basic nature hence it is not amphoteric nature.
Is H2CO binary or ternary?
A binary compound is a compound in which the molecule is formed by two atoms out of which one atom is hydrogen which combines with another atom. Also, a ternary compound is a compound in which the three different are combined to form a molecule, Therefore, in the case of H2CO molecule is formed by three different atoms which are hydrogen, carbon and oxygen. Hence this molecule is a ternary compound.
Is H2CO balanced?
Yes, H2CO can form a balanced equation when reacting with sodium hydroxide also when reacting with ammonia it will form a balanced equation, the reaction is said to be balanced when both reactant and product have an equal number of atoms.
2 H2CO + NaOH – HCOONa + CH3OH
In the above reaction, formaldehyde reacts with sodium hydroxide which forms sodium fumarate and methanol. In this reaction, the numbers of atoms are equal in both the reactant and product hence this reaction is balanced.
6HCHO + 4NH3 → (CH2)6N4 + 6H2O
In the above reaction when the formaldehyde is reacted with ammonia, it forms formamide and water this reaction is also balanced because both reactant and product contain an equal number of atoms.
Is H2CO conductive?
Yes, H2CO is conductive. Formaldehyde when dissolving in the water gets ionized and forms ions as HCO– and H+ ions, thus it behaves as a weak electrolyte. The molecule is said to be conductive when it is dissociated into its ions by dissolving the molecule in an aqueous solution. Hence H2CO is a weak electrolyte and conducts electricity through its ions.
Is H2CO conjugate base?
No H2CO is not a conjugate base. But it can produce a conjugate base by donating its H+ ion. According to the Bronsted-Lowry theory of acid and base, when the molecule donates an H+ ion then it is Bronsted acid and when the molecule accepts an H+ ion then it is called Bronsted base.
Why H2CO can form a conjugate base?
Formaldehyde (H2CO) when dissolve in water it dissociates into hydronium ion (H3O+) and HOC conjugate base by releasing its H+ ion or proton.
H2CO+H2O – H3O++ HOC
Is H2CO corrosive?
Yes, formaldehyde (H2CO) is corrosive in nature. Vapours of formaldehyde are flammable when it is exposed to heat or flame then it becomes an explosion. Formaldehyde (H2CO) may irritate the eye and skin. It can cause serious or permanent injury.
Is H2CO concentrated?
Yes, formaldehyde (H2CO) is concentrated because it is a naturally occurring organic aldehyde.
Is H2CO solid liquid or gas?
Formaldehyde is in the gaseous form. when it is in an aqueous solution like water then it acts as an organic liquid.
Is H2CO hygroscopic?
Yes, formaldehyde (H2CO) is hygroscopic in nature. It is a gas which absorbed water from the surrounding and form a formalin solution, hence it is hygroscopic in nature. The molecule which absorbs water from the surrounding or air is called hygroscopic in nature.
Is H2CO hydrogen bonding?
In Formaldehyde molecule H2C=O. The oxygen atom has lone pair which is a receiver, that can form a hydrogen bond with another molecule such as water. H2C=O —– H2O, But formaldehyde does not form hydrogen bonding with another formaldehyde molecule, because hydrogen bonding requires doner usually -OH & -NH group or receiver usually lone pairs, H2CO has lone pair as a receiver but it does not contain doner hence it is not participating in hydrogen bonding with another formaldehyde molecule.
Is H2CO metal or nonmetal?
Formaldehyde (H2CO) is nonmetal in nature. All the atoms in the H2CO molecule are carbon, hydrogen and oxygen all are nonmetals hence formaldehyde is a nonmetal. It is in gaseous form hence nonmetal in nature.
Is H2CO neutral?
H2CO is a neutral molecule. The atoms present in the formaldehyde molecules have zero partial charges, also it does not contain any partial positive or negative charge, hence formaldehyde (H2CO) is a neutral molecule.
Is H2CO a nucleophile?
Nucleophiles are the species which are formed bonds by donating electron pairs. The molecules or ions with a free pair of electrons or at least one pi bond act as a nucleophile, H2CO act as a nucleophile because it has two lone pairs on the oxygen atom, which can form a bond by donating that free lone pair hence it acts as a nucleophile.
Is H2CO organic or inorganic?
Formaldehyde (H2CO) is an organic compound. Organic molecules are those molecules which contain carbon-hydrogen or carbon-carbon bonds. Hence formaldehyde has a carbon atom also it forms a carbon-hydrogen bond in between them hence it is organic in nature.
Is H2CO oxidizing agent?
Oxidizing agents can gain or accepts electrons from the other atom or molecule while reducing agents can lose or share electron pair with another atom or molecule. Formaldehyde (H2CO) molecule can share electron pair with another molecule or atom. Also, it has CHO (aldehyde) group hence it is not an oxidising agent.
Is H2CO polyatomic?
Yes, formaldehyde (H2CO) is a polyatomic molecule because its structure contains more than two atoms that are two hydrogens, one carbon and one oxygen atom. hence H2CO is polyatomic in nature.
Is H2CO unstable?
No formaldehyde (H2CO) is a stable organic gaseous molecule, it is stable at 150oC, and it polymerizes when condensed to a liquid. decomposes
Is H2CO volatile?
Yes, formaldehyde is a volatile organic compound. because formaldehyde does not form hydrogen bonding with the other molecule hence it is highly volatile in nature.
Is H2CO viscous?
Pure formaldehyde is in a gaseous state hence it is not viscous. While it is in aqueous solution it has 2.083- 2.835 mPa at 200C.
In the above article we can discuss the Lewis structure of formaldehyde (H2CO), and its various characteristics in detail. like hybridization , shape, lone pair , valence electrons , solubility, acidic or basic nature, boiling point , bond angle and so on.
Also Read: