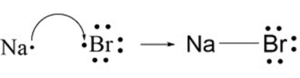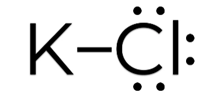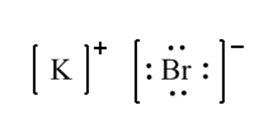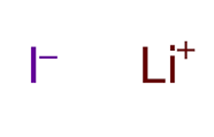Reflection is an essential phenomenon in physics. This article will find out the different Reflection of light examples.
Reflection of light is a process that gives us the ability to look at the features around us. The joint Reflection of light examples of light around us differentiates the colors, images, camera, etc. The light waves undergo a specific process of laws of Reflection and finally reflect the image of the object to be observed.
In this article, we will study the various Reflection of light examples.
Reflection of light examples
The numerous Reflections of light examples that we find around us are below.

- Mirror images
- The image formed by Reflection through the spherical mirror
- Reflection through headlights
- Reflection through side mirror of vehicles
- Reflection of the image in water bodies
- Reflection through shoe polished surface
- To observe various colors around us
- Working of the camera through Reflection of light
- Working of Microscopes through Reflection of light
- Working of Telescopes through Reflection of light
- Reflection of light on a polished rough surface
- Reflection of light in formation of images through eyes
- Shining of beautiful stars
- Reflection observed in luminous bodies
- Reflection of light observed in moon
- Reflection observed in non-luminous bodies
Mirror images
The mirror images are one of the daily Reflection of light examples. We see our self-reflection in mirrors to know we look when we get ready to go out somewhere. This Reflection is possible when the light in the process of Reflection obeys the law of Reflection. The incident light rays hit an angle and reflected in the opposite direction in the same path. It leads to the formation of any image.

The image formed by Reflection through the spherical mirror
The term spherical makes us think about the three-dimensional circle shapes. The spherical mirror usually consists of a polished surface. Still, suppose you are curious to know how the Reflection happens when divided into two halves. In that case, we get the other two mirror types: the inner part will resemble a concave mirror, and the outer part of each half will resemble the convex.

Since the spherical mirror has a polished surface, the light incident on it gets reflected in the form of image; it seems almost the same as a plane mirror reflection, but there are some complications since it includes convex and concave.
Reflection through headlights
The mirrors through which headlights are designed are concave. Before knowing how a reflection from a concave type mirror is an example of Reflection of light, we have to know the characteristics.
The Reflection takes place similar to a plane mirror, and it has two surfaces, a polished and a stable surface. The outer part will be polished, and the inner one will be normal, so the Reflection takes place from any inner point of concave type objects. So as the light waves get incident on the inner part of concave headlights, it produces a reflection with satisfying the laws of Reflection.

Reflection through side mirror of vehicles
The side mirrors of the vehicles are mainly constructed using a convex lens material. The necessary characteristic of a concave mirror is that Reflection usually takes place from all the points of the outer part because it is not polished. At the same time, the inside surface does not reflect any light since it is polished. With the help of the reflection phenomenon, the side mirrors help us see the objects that are approaching.

Reflection of the image in water bodies
Whenever we go to a picnic near any water bodies, kids get excited to see their self-images in the water. The formation of images in the water bodies is possible due to the process of Reflection; a certain amount of light waves from us act as incident ray and approaches the water bodies. Here the light rays get reflected, leading to the formation images. The same phenomenon can be seen in any water source in a glass or large water body.

Reflection through shoe polished surface
Whenever a reflection occurs through a polished surface, it will satisfy the laws of Reflection. This shiny Reflection can be easily observed when you polish your shoes using a lubricant, the shiny surface through which we can see the images takes place due to the process of Reflection. Therefore, it is one of the best Reflection of light examples. The other polished surfaces are steel materials or any polished surfaces.

To observe various colors around us
We observe many colors around us, from the rainbow to small objects; everything is composed of different colors. These different colors are visible and observed with the reflection process’s help. The objects such as flowers, animals, insects, and birds emit particular light that gets Reflection back and helps in color formation on their body.

Working of the camera through Reflection of light
Nowadays, professional photography is one of the most economical jobs. The use of the camera to click beautiful pictures undergoes a process of Reflection to produce those images. There will be the presence of more minor inside a camera; when the incident light from the desired image falls on the lens, it gets reflected in producing the high quality of the image. Therefore, the camera’s work is an excellent Reflection of light examples.

Working of Microscopes through Reflection of light
The same principle of Reflection seen in the camera lens’s working is observed in microscopes. Even the microscope consists of a lens that helps produce the image required to be observed. The microscope is, in general, used in labs to observe many tiny creatures or phenomena in physics. The incident light from the slide of the image falls on the microscopic lens that reflects the image of the slide. It is an essential reflection of light examples seen in labs.

Working of Telescopes through Reflection of light
Telescopes are used to see far objects, and it is one of the best Reflection of light examples widely used in space laboratories—the same principle of Reflection that is seen in the working of the telescope lens. The telescope consists of lenses that help produce the image by undergoing multiple reflections that help observe the focused images. The incident light from the body of the image to be observed strikes on the lens present in the telescope, reflecting tit, producing a clear image.

Reflection of light on a polished rough surface
When an image is produced from any polished rough surface, we can ensure that the light rays have undergone Diffuse Reflection. Polishing makes the reflection rays inhibit and later emit properly, gradually increasing the scope for Reflection. The Reflection from these surfaces will be an authentic reflection of light examples.
Reflection of light in formation of images through eyes
- The eyes are an essential body part for every human. Without eyes, it would not have been possible to grasp the beautiful environment around us. We cannot see any object or body in a very dark room due to the absence or lack of light sources. So, both light and eyes are interrelated, informing the images with the help of Reflection.
- The reflection process in the eyes is explained as follows. When we see any object a certain amount of reflected light from, any material strikes the lens present in our eyes. It will lead to the formation of virtual and inverted images in the region of the retina.
- The signal from the retina is transferred to the brain cell that leads to Hence formation of an image through eyes are fundamental Reflection of light examples.

Shining of beautiful stars
Stars are one of the beautiful creations of the universe, and we feel joy when we look at the pack of shining stars at night. We observe some discrimination when we notice that some stars shine brightly where the other stars will be diminished. The reason behind it is the intensity of reflected rays.
The stars generally come under the category of luminous bodies, and they will be generating their light. A certain amount of Reflection that strikes from the stars makes them visible.

These stars emit certain light rays into the space that will gradually come into the earth’s atmosphere; it will act as incident light that hits the earth’s surroundings and strikes back again to them, leading to the shining property of stars.
Reflection observed in luminous bodies
The main asset of the phenomenon of Reflection is considered in luminous bodies; they are the actual creatures that produce light and give it to non-luminous creatures. The primary examples of luminous bodies are stars, the sun, and the unique insect called fireflies.
These bodies produce light rays that fall on the non-luminous creatures. The incident beam ray falls on objects, and the obtaining of reflected rays leads to the formation of images. The other luminous bodies, such as a torch, lamp, candle, etc., are observed.

Reflection of Light observed in the moon
Moon is a significant celestial body that is very close both physically and emotionally in the mind of people. The beautiful moon is the best example of a non-luminous object. It has no light waves of its own, and it seeks light from the sun; this light is reflected through the moon to our eyes helps in getting the full image of the moon. The nature of Reflection seen here is diffuse since the surface on the moon have irregularities and is one of the vital Reflection of light examples of nature.

To know more: Diffuse Reflection
Reflection observed in non-luminous bodies
Reflection is also seen in non-luminous bodies; the unique feature of these bodies is that they don’t have the light of their own. But how these objects are visible is a question to think about. These bodies gain light rays from luminous bodies and gradually develop their light that undergoes reflection process, seeing them. The non-luminous bodies such as our earth, sheets, paper, plants, etc., are observed through this process.

These are some essential and precise reflections of light examples in nature.
Frequently Asked Questions | FAQs
What is the actual meaning of Reflection of light?
The phenomenon of Reflection of light signifies how everything around us is visible to us in different colors.
The meaning of Reflection also indicates that self-images are observed with the help of light. In this process, a particular beam of the incident called the initial wavefront of light strikes on a particular medium and comes back in the opposite direction with the same intensity. The light ray or wavefront that comes back in the opposite direction is reflected. Both the rays of light will be straight in nature.
Mention the different applications of Reflection of light?
The critical and different applications of Reflection of light examples that help in leading smooth life is as shown below,
- Working of microscope
- Working of telescope
- Mirrors present in the vehicle
- Working of Kaleidoscope
- Torchlight
- Street Light
- Helps in identifying the minor mistakes using a microscope
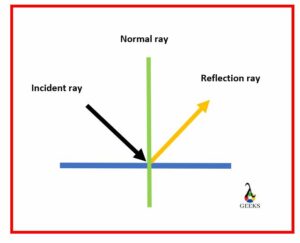
What are the main aspects of the laws of Reflection?
The main aspects of laws of Reflection that must be satisfied by the beam of light wavefronts for proceeding the process of Reflection are mentioned below,
- When any beam of light rays falls on a surface without irregularities, the angle at which light strikes the surface must always be equal to the angle at which it gets reflected, also known as a reflected ray.
- All the three rays, the initial ray, the reflected ray, and the point at which both the rays strike and emit perpendicular called ordinary ray, always lie in the same plane.
What are the examples of good reflectors of light?
If the surface of the object produces a maximum reflection, then those materials are considered good reflectors of light. Some of the best reflectors of light are listed below,
- Water sources
- Mirrors (Plane, Spherical, concave, convex)
- Steel metals
- Shining surfaces
- Glass
- Light-colored materials
Also Read:





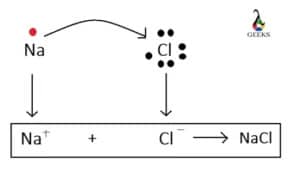
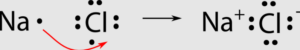
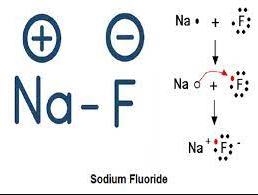
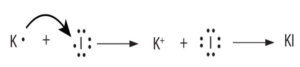
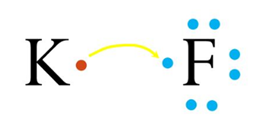
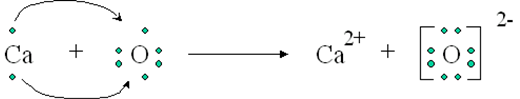
 A+ + B– A is a cation with a positive charge and B is an anion with a negative charge.
A+ + B– A is a cation with a positive charge and B is an anion with a negative charge.