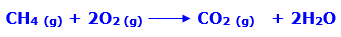
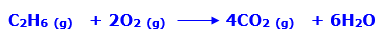
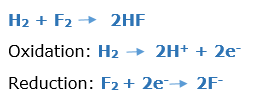
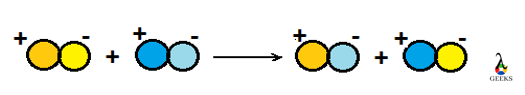
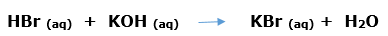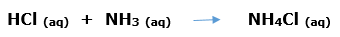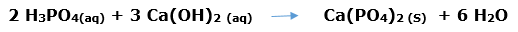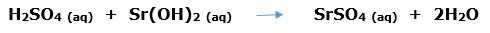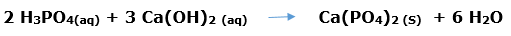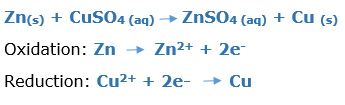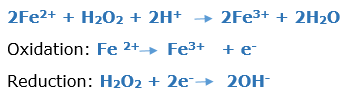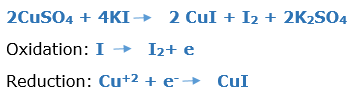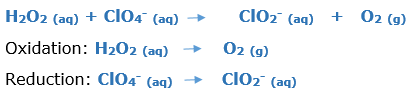In this article, we are going to see precipitation reaction examples with detailed explanations.
- Silver nitrate AgNO3 and Potassium chloride KCl
- Ferrous sulphate FeSO4 and Sodium hydroxide NaOH
- Silver nitrate AgNO3 and Sodium chloride NaCl
- Calcium chloride CaCl2 and Potassium hydroxide KOH
- Magnesium hydroxide Mg(OH)2 and Hydrochloric acid HCl
- Sodium hydroxide NaOH and Copper sulphate CuSO4
- Strontium chloride SrCl2 and Sodium sulphate Na2SO4
- Lead nitrate Pb(NO)3 and Potassium iodide KI
- Ferric sulphate Fe2(SO)4 and Sodium hydroxide NaOH
- Potassium sulphide K2S and Cadmium sulphate CdSO4
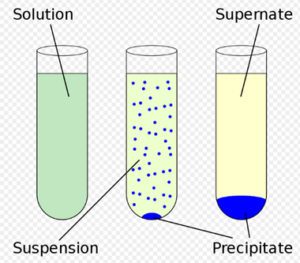
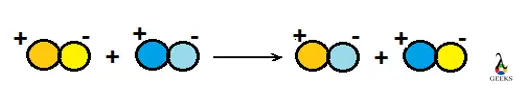
The chemical reaction in which two ions combined to form one of the insoluble products in an aqueous solution that precipitated out. The precipitate is shown by the down arrow in the chemical reaction. It is used to check out elements present in the solution. It is a type of double displacement reaction.
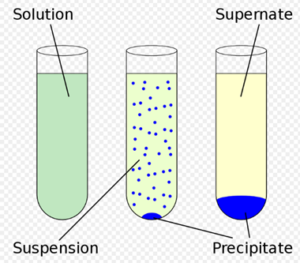
Image Credits : Wikipedia
Also Read On: 15 Coordinate Covalent Bond Examples: Detailed Insight And Facts
Precipitation Reaction Examples
Silver nitrate and Potassium chloride
The insoluble salt or precipitate of silver chloride AgCl is a product of the reaction between Silver nitrate AgNO3 and potassium chloride KCl. The silver chloride AgCl is a water-insoluble, solid, and white colour precipitate. The reaction occurs in an aqueous solution. So that ions get formed to replace each other and to form the salt.

Ferrous sulphate and Sodium hydroxide
The iron (ll) hydroxide Fe(OH)2 is an insoluble salt formed when ferrous sulphate FeSO4 and sodium hydroxide NaOH undergo a reaction. Sodium sulphate Na2SO4 is formed as a co-product. Iron (ll) hydroxide is a green coloured solid.

Silver nitrate and Sodium chloride
The insoluble salt or precipitate of silver chloride AgCl is a product of the reaction between Silver nitrate AgNO3 and sodium chloride NaCl. Sodium nitrate is also formed as a co-product. The silver chloride AgCl is a water-insoluble, solid, and white colour precipitate. The reaction occurs in an aqueous solution. So that ions get formed to replace each other and to form the salt.

Calcium chloride and Potassium hydroxide
The calcium hydroxide Ca(OH)2 is precipitated out by the reaction of calcium chloride CaCl2 and potassium hydroxide KOH. The reaction takes place in an aqueous medium only. The calcium hydroxide Ca(OH)2 is a water-insoluble, solid, and white colour precipitate.

Magnesium hydroxide and Hydrochloric acid
The magnesium chloride MgCl2 is an insoluble salt formed when magnesium hydroxide Mg(OH)2 and hydrochloric acid HCl react with each other. The magnesium chloride MgCl2 is a colourless or white solid and water formed as a co-product.
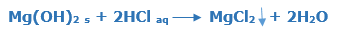
Sodium hydroxide and Copper sulphate
The copper hydroxide Cu(OH)2 is precipitated out when sodium hydroxide NaOH and copper sulphate CuSO4 react with each other. The copper hydroxide Cu(OH)2 is a blue colour solid and Sodium sulphate formed Na2SO4 as a co-product.
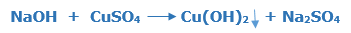
Strontium chloride and Sodium sulphate
The strontium sulphate SrSO4 is water-insoluble salt, that precipitate out when strontium chloride SrCl2 and sodium sulphate Na2SO4 react with each other. Sodium chloride NaCl is also formed as a co-product. The strontium sulphate SrSO4 is a water-insoluble, solid, and white colour precipitate.

lead nitrate and Potassium iodide
The lead iodide Pbl2 is precipitated out by the reaction of lead nitrate Pb(NO)3 and potassium iodide KI. Potassium nitrate KNO3 is formed as a co-product. The reaction required aqueous conditions. The lead iodide Pbl2 is yellow coloured solid.

Ferric sulphate and Sodium hydroxide
The iron (lll) hydroxide Fe(OH)3 is an insoluble salt formed when ferric sulphate Fe2(SO)4 and sodium hydroxide NaOH undergo a reaction. Sodium sulphate Na2SO4 is formed as a co-product. Iron (lll) hydroxide is brown coloured solid.

Potassium sulphide and Cadmium sulphate
The cadmium sulphide CdS is formed by the reaction of potassium sulphide K2S and cadmium sulphate CdSO4. The potassium sulphate is formed as a co-product. Cadmium sulphide CdS is a yellow coloured solid, water-insoluble. The reaction required an aqueous medium.

Also Read On: Alkyl Halide Examples: Detailed Insights And Facts
Facts
- The chemical reaction in which two ions combined to form one of the insoluble products in an aqueous solution that precipitated out.
- The precipitate is shown by the down arrow in a reaction.
- It is a type of double displacement reaction.
- It affects by the type and size of ions, the concentration of an aqueous solution, pH of the solution, solubility etc.
Frequently Asked Questions:
Question: What is a precipitation reaction?
Answer: The precipitation reaction is,
The chemical reaction in which two ions combined to form one of the insoluble products in an aqueous solution that precipitated. The precipitate is shown by the down arrow in the chemical reaction. It is used to check out elements present in the solution. It is a type of double displacement reaction.
Question: What are the factors that affect the precipitation reaction?
Answer: Factors affecting precipitation reaction are,
Type and size of ions, the concentration of an aqueous solution, pH of the solution, solubility etc.
Question: What are examples of precipitation occur in nature?
Answer: The precipitation occurs in nature,
The most common examples of precipitation are Rain, Snowfall, hail, sleet, dew etc.
Also Read On: 5+ Metallic Bond Examples: Explanation and detailed Facts