How to Find Redox Reaction

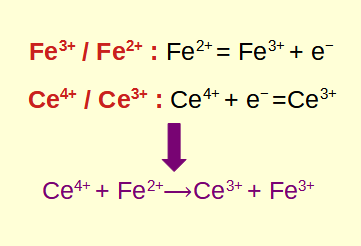

redox reactions, short for oxidation-reduction reactions, are fundamental chemical processes that involve the transfer of electrons between reactants. Understanding how to identify and calculate redox reactions is crucial in the fields of chemistry and biology. In this blog post, we will explore the basics of redox reactions, learn how to identify them, delve into the calculations involved, and explore their practical applications.
Understanding the Basics of Redox Reactions
Redox reactions occur when there is a transfer of electrons between two species. One species loses electrons (undergoes oxidation) while the other species gains electrons (undergoes reduction). This transfer of electrons is driven by a difference in the electronegativity of the atoms involved.
The oxidized species is known as the reducing agent because it donates electrons, while the reduced species is called the oxidizing agent because it accepts electrons. It’s important to note that redox reactions always involve both oxidation and reduction, hence the name.
Importance of Redox Reactions
redox reactions play a crucial role in various chemical and biological processes. They are involved in energy production, corrosion, synthesis of chemicals, and even the functioning of our own bodies. For instance, the process of oxidative phosphorylation, which occurs in our cells’ mitochondria, relies on redox reactions to produce ATP, the universal energy currency of cells.
Common Examples of Redox Reactions
Let’s take a look at some common examples of redox reactions:
Combustion: When a substance reacts with oxygen to produce heat and light, it is a redox reaction. For example, the burning of wood or the combustion of gasoline in a car engine.
Rusting: The process of rusting involves the oxidation of iron in the presence of oxygen and water. The iron loses electrons, forming iron(III) oxide (rust).
Photosynthesis: During photosynthesis, plants convert carbon dioxide and water into glucose and oxygen, with the help of sunlight. This process involves the reduction of carbon dioxide and the oxidation of water.
Identifying Redox Reactions
To identify a redox reaction, we need to recognize the substances that are undergoing oxidation and reduction. Here are a few key steps to help us identify redox reactions quickly:
Recognizing oxidation and reduction: Look for changes in oxidation states or the loss/gain of electrons. If an element’s oxidation state increases, it is being oxidized, while a decrease in oxidation state indicates reduction.
Determining the oxidation Number: The oxidation number is a concept used to determine the distribution of electrons in a compound or ion. It helps us identify the change in oxidation states during a redox reaction.
Identifying redox reactions Quickly: One effective method to identify redox reactions is by looking for the presence of certain elements or compounds known to undergo redox reactions. For example, hydrogen peroxide (H2O2) is a common oxidizing agent, while substances like sodium borohydride (NaBH4) are reducing agents.
Calculating Redox Reactions
Once we have identified a redox reaction, we can proceed with calculating it. Here’s how:
Finding the Half reactions: Split the redox reaction into two half-reactions, one representing oxidation and the other representing reduction. This helps in balancing the equation later on.
Balancing the redox Reaction: Balance the number of atoms on each side of the equation by adjusting coefficients. It’s essential to balance both mass and charge when dealing with redox reactions.
Calculating the Overall Redox Reaction: Combine the balanced half-reactions, ensuring that the number of electrons gained in reduction matches the number of electrons lost in oxidation.
Advanced Concepts in Redox Reactions
To deepen our understanding of redox reactions, let’s explore some advanced concepts:
Determining Spontaneity of redox Reactions: The spontaneity of a redox reaction can be determined using the concept of redox potential. If the redox potential is positive, the reaction is spontaneous, indicating a higher likelihood of occurring.
Finding the Coefficient of redox Reactions: The coefficients in a balanced redox reaction represent the relative number of moles of each species involved. These coefficients are crucial for stoichiometric calculations.
Understanding the Role of Electrons in redox reactions: Electrons are the carriers of charge in redox reactions. They travel from the reducing agent to the oxidizing agent, facilitating the transfer of energy.
Practical Applications of Redox Reactions
Redox reactions have practical applications in various domains. Let’s explore a few examples:
redox reactions in Everyday Life: The process of respiration in our bodies relies on redox reactions to convert glucose into energy. Additionally, the batteries we use daily, such as alkaline batteries and lithium-ion batteries, operate based on redox reactions.
Industrial Applications of Redox reactions: Redox reactions are crucial in industrial processes like metal extraction, electroplating, and wastewater treatment. They help remove harmful pollutants and convert them into less toxic substances.
redox reactions in Biological Systems: In the human body, redox reactions are involved in various biological processes, including metabolism, detoxification, and immune responses. However, excessive redox reactions can lead to oxidative stress, which can damage cells and contribute to diseases.
Also Read:
- Knoevenagel reaction
- Light dependent reaction example
- Endothermic reaction
- Nucleophilic substitution reaction
- Synthesis reaction example
- Photochemical reaction
- Endergonic reaction example
- First order reaction example
- Light independent reaction example
- Kolbe reaction

Hello,
I am Aditi Ray, a chemistry SME on this platform. I have completed graduation in Chemistry from the University of Calcutta and post graduation from Techno India University with a specialization in Inorganic Chemistry. I am very happy to be a part of the Lambdageeks family and I would like to explain the subject in a simplistic way.
Let’s connect through LinkedIn-https://www.linkedin.com/in/aditi-ray-a7a946202
