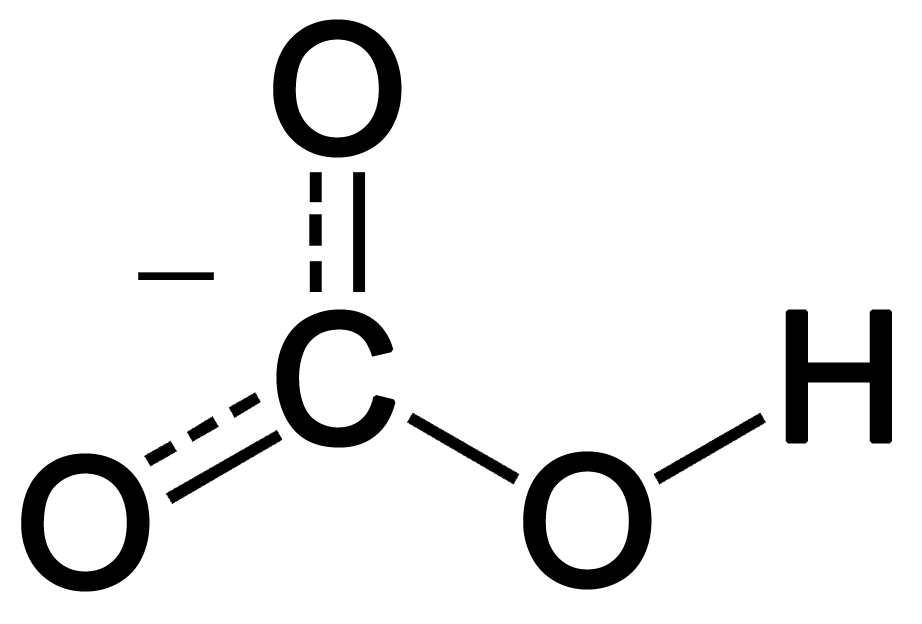
HCO3- Lewis structure is reliable in denoting considerable chemical and physical properties of Bicarbonate. As Lewis structure brings forth a fundamental sketch of HCO3-, it is effective in highlighting the electronic fact about the compound.
HCO3- Lewis structure and the characteristics of this organic compound will be presented in a well-structured manner through this article. Several physical properties and chemical facts will be illustrated in this study to clarify the significance of drawing its Lewis structure.
Drawing HCO3– Lewis structure
Drawing Lewis structure of Bicarbonate ion (HCO3-) is quite easy. This Lewis structure refers to the electronic structure of the compound imposing the sharing process. It highlights choice of central atom and the bond type generated by the shared electrons. Lewis structure formation is followed by few easy steps. The systematic progression of the electron share procedure helps to identify some chemical facts about HCO3-.
Step 1: Finding the number of valence electrons present in the element participating in the formation of HCO3- ion is the fundamental step of drawing Lewis structure.
Step 2: Second step highlights the calculation of bond pairs that are assembled by the elements by sharing their valence electrons with each other.
Step 3: In this step the atom that is capable of holding the centre position is found by evaluating its electronegativity and number if participated atoms. In HCO3- ion, Carbon holds this position as electronegativity of Hydrogen is lower than carbon. Oxygen cannot stay in middle as three atoms participate in bonding.
Step 4: The fourth step significantly process the skeleton of the Lewis structure by determining the position of atoms. Connecting the paired electrons of different atoms by Sigma and Pi bonds in HCO3 is done in this step.
Step 5: Putting the other remaining electrons of oxygen around the atoms in the structure completes the whole Lewis structure. The electrons are denoted by dots.
HCO3- Lewis structure resonance
Lewis structure of compounds containing negative ions and pi bonds are reliable developing more than one resonating structure. Resonance take place in a compound due to the tendency of extra negative ion to create pi binds by breaking another pi bond present in the compound.
In HCO3-, one oxygen atom creates double bond with Carbon and another two create single bonds with the same and one of those hold a negative charge with the presence of extra electron. That electron influences the compound to impose resonance.
HCO3- Lewis structure shape
The position of central atom and bond angle are the factors that determine the shape of the compound. Lewis structure is reliable sketch of compounds, which insists information about shape of the compounds.

According to VSEPR (Valence Shell Electron Pair Repulsion) theory, the shape of Bicarbonate ion is Trigonal planner. The compound to minimize the problem of electron pair repulsion obtains this shape.
HCO3- Lewis structure formal charge
Lewis structure of compound identifies the formal charge of the individual elements participate in the formation of the compound. There is a specific formula followed by the chemists to identify the formal charge of individual elements.
The formula is Formal charge = Number of valence electrons – Number of nonbonding valence electrons – (Bonding electrons/2)
Formal charge of carbon = (4-0-(4/2)) = 2
Formal charge of Oxygen with negative charge = -1
Formal charge of other two oxygen = (7-6-(1/2)) =0.5
Formal charge of Hydrogen = (1-0-(1/2)) = 0.5
The above calculation is exposing that this large size ion has a net charge of -1.
HCO3- Lewis structure angle
The shape or geometry of the compounds is responsible for recognising bond angle held by the overall structure. Lewis structure initiates the process of identifying the angle of between the bonds created by element through electron share.
An ideal angle of 120° is possessed by Bicarbonate ion. HCO3- has ben obtained with an idea shape of Triginal planner that denotes that the compound has 120° of bond angle.
HCO3- Lewis structure octet rule
Octet rule is key driver for manipulating the elements to undergo electron-sharing mechanisms. This rule executes the fact that each element in the periodic table wants to adopt eight electrons in its last energy level to achieve ultimate stability like their nearest noble gas element (Such as Helium, Argon, Redon, Xenon and Krypton).
Octet rule is fulfilled by the compound through donating the extra electron from valence shell of adopting electrons from other electrons to pack the deficiency of electron. In HCO3-, carbon, the central; atom shares its four electrons with oxygen atoms an adopt four electrons from them to make the last energy level filled with eight electrons.
HCO3- Lewis structure lone pairs
Detection of the presence of the lone pairs in the compounds is supported by Lewis structure, as this structure is reliable in implementing number and position of electrons in the compound.
Bicarbonate ion contains lone pair on oxygen atoms only as all the eight electron pairs of carbon are bonded. In right oxygen, two lone pairs and in left oxygen three lone pairs are present. The oxygen attached with hydrogen also contain two lone pairs only. Therefore, the total number of lone pairs present in the structure of HCO3- is seven.
HCO3- valence electrons
Calculating the number of valence electrons is the most important factor for each atom to identify its deficiency or excesses of electrons. This calculation initiates the process of drawing Lewis structure of a compound.
The number of valence electron present in Carbon is four, in each of the oxygen it is seven. Hydrogen holds one valence electron. The total number of valence electron in HCO3- is (4+(3*7)+1) = 26.
HCO3- hybridization
The presence of lone pairs and bond pairs determine the hybridisation of the compounds. Geometric shape of the compounds is obtained from the Lewis structure of ions or compounds. This is a feature relates the fact of hybridisation.
Sp2 hybridisation is notice in HCO3- ion. The number of lone pairs on the central atom (C) is zero and it has three sigma bonds with steric number of three. These criteria indicate sp2 hybridisation of Bicarbonate ion.
HCO3- solubility
Solubility of ions is highly dependent on the charge contained by the ions. Density of charges and energy inside the compounds are significant factor to identify solubility of the compounds.
HCO3- is highly soluble in water and slightly soluble in solvent containing OH (Hydroxyl) group. The bicarbonate salts are insoluble in acidic solvents.
HCO3- soluble in water
A single anion cannot be soluble in water it should always be bonded with a cation and form salt to show solubility in water. Bond strength is the feature, which determines the soluble nature of a compound or salt.
Maximum salts with Bicarbonate ion are highly soluble in water. Carbonates such as Ca(HCO3), Mg(HCO3) and many more are quite soluble in water.
HCO3- an electrolyte
Goof dissociation ability in solution refers to be a good electrolyte. After dissociating into separate ions, it helps the solution to conduct electricity superiorly, which is considered as most viable property of an electrolyte.
Bicarbonate is an electrolyte (22-29 mmol/L) as it displays the dissociation of H+ ion from the complex ionic structure. This negatively charged ion is helpful for maintaining pH balance in body and conduct electricity in molten state as well.
HCO3- a strong electrolyte
Bicarbonate ion is no doubt an electrolyte as it is capable of leaving H+ ion. giving out free ion can incorporate ability in the compounds to shoe competency as electrolyte by making them conduct electricity.
Conjugate acid of HCO3- (H2CO3) and Bicarbonate ion both are not strong electrolyte as the existence of free H+ ions in molten state is unstable which makes it is a weak base as well.
HCO3- acidic or basic
Acidic or basic nature of compounds or ions depends on the factor of having H+ and OH- ions. The number of H+ and OH- determines pH level of the compound that is supposed to describe the nature of the compound or ion.
HCO3- contains both H+ and OH- ions which denotes hybrid nature of the ion. Bicarbonate ion is generally noticed to be basic in nature but sometimes is exposes acidic behaviour as well.
HCO3- a strong acid
Strength of acidity depends on the free movement of H+ ions in a compound. in HCO3- the H+ ions do not impose free movements in solution which is refer to opposite characteristic for being a strong acid.
HCO3- is a weak acid as well as weak base. Naturally, it does not show dramatic change in pH level after being soluble in water. The H+ ions attached with bicarbonate ion, which highlights its basic appearance. Hydrogen ions do not get dissociated in a huge amount which relies on the fact that the ion is a weak acid as well.
HCO3- polyprotic acid
Polyprotic acids refer to those acids which are capable of donating more than one proton (H+). Capacity of donating two or three or more than three protons respectively makes Polyprotic acids distinguishable from Diprotic and Triprotic acids.
HCO3- is basically a weak base by nature still its acid form can donate one H+ but not more than that. Therefore, Bicarbonate ion cannot be considered as Polyprotic acid rather it can be presumed that it has a few potentiality in exposing Monoprotic nature.
HCO3- a Lewis acid
A Lewis acid refers to the elements which contain empty orbitals where it can accept electron pairs. Lewis acids has ability of accepting electron whereas Lewis bases are capable of donating electrons.
Bicarbonate is a Bonsted-Lowry acid, which cannot accept electrons. It is able to accept proton from HCL to form its conjugate acid that is carbonic acid, H2CO3. Therefore, HCO3- is not a Lewis acid.
HCO3- an Arrhenius acid
Arrhenius acids are those elements, which can readily lose protons (H+). Only criteria for losing H+ is that the element must be in molten state that is on dissociation in water that could release H+ ions.
Bicarbonate ion has been identified to donate one H+ ion at a time in molten state and form CO32- ion. Besides, the ion is active in donating OH- ion as well to give out CO2. Therefore, it can be considered as both Arrhenius acid and base.
HCO3- polar or nonpolar
Polarity depends on shape and bonding of the compounds. On the other hand difference between electronegativity of the elements also delivers a certain amount of polarity to the compounds.
In HCO3- the elements have huge difference in electronegativity and the shape of the compound is not symmetric as well. Therefore, a dipole-dipole interaction takes place among the elements, which makes it polar by nature.
HCO3- linear
Linear shape can be noticed on those compounds where two atoms are attached with one central atom and the angle of the compound is noticed to be 180°. Horizontal alignment is present in the linear compounds.
HCO3- is absolutely differ from linear its central atom, carbon holds three oxygen atoms around it. One oxygen is attached with double bonds and another two creates single bond with carbon.
HCO3- paramagnetic or diamagnetic
Presence of only unpaired electrons in any compound makes it diamagnetic whereas presence of only one unpaired electron refers to paramagnetic nature of a compound.
HCO3- is neither diamagnetic not paramagnetic as all the electrons in the compound are paired it has total 12 pairs of electrons where eight pairs are belonging from lone pair category.
HCO3- boiling point
the particular temperature at which a compound can change its liquid state into vapour is called the boiling point of that compound.
Bicarbonate ion itself cannot show physical property of boiling. When it is conjugated with any metal such as Sodium the overall molten state of that compound can impose a specific boiling point that is 851°C.
HCO3- bond angle
Lewis structure is a valid factor that reveals the angle of the bonds in any compound. Besides, VSEPR theory also effectively insists the fact of holding suitable bond angle adopted by the composite structure of elements.
The bond angle of Bicarbonate ion (HCO3-) has been identified120° through VSEPR theory. This theory says that this HCO3-wants to cut off the effect of lone pair lone pair and lone pair repulsions from its geometry. Therefore, for having a stable Trigonal Planner shapes with the angle of 120°.
HCO3- diprotic
Diprotic acids are those acids, which contain two proton or H+ ions as an important part of compounds. Carbonic acid is a great example of Diprotic acids, it hold two H+ ions and can donate one to gives out HCO3-, Bicarbonate ion.
HCO3- is not diprotic as it has only one proton, which is not even readily donated by the ion rather than in molten state.
HCO3- ionic or covalent
When the atoms permanently donate their electrons to another atoms they make ionic bonds, when partially electron share takes place amid atoms the they form covalent bonds. According to tis bonding abilities the chemical nature of compounds are judged in chemistry.
Bicarbonate ion is formed by Hydrogen, oxygen and carbon by sharing valence electrons with each other partially to fill octet state. It helps them to generate sigma bonds with covalent structure. Therefore, the complex ion can be considered as a covalent compound.
HCO3- amphiprotic
Water is a great example of amphiprotic compound, which refers to the property of both accepting and donating proton. Water can Release H+ and OH- ions both Similarly HCO3- is also capable of donating both H+ and OH- ions.
Bicarbonate ion is able to accept and donate H+ ions, which deliberately impose the information that this ion is amphiprotic like Water. By losing proton, it gives carbonate ion and gaining the same it gives carbonic acid.
HCO3- a conjugate acid or base
According to Bronsted-Lowry Acid-base theory, when an acid donate one or more protons to a base it is considered as Conjugate acid of that particular base. Similarly, when a base loses its hydrogen ions as a reverse reaction that is called conjugate base.
Bicarbonate is a conjugate base of carbonic acid as when carbonic acid loses it Hydrogen ions HCO3- ion forms readily. Besides, when CO32- is formed from dissociation of H+ ion from acid HCO3-.
HCO3- a proton donor
Proton donor refers to that compounds which are good donor of H+ ions. According to chemical facts of carbonic acid, it can be said that Carbonic acid is able to lose proton and produce HCO3- ion fluently. Therefore, H2CO3 is a proton donor.
Acid HCO3- also loses proton (H+) in molten state but cannot be stabilised in the dissociated form for long which makes it’s a bad proton donor. It works as good conjugate base.
HCO3- an electrolyte
Bicarbonate ion is reliable in highlighting its ability of losing free H+ electrons. Though the free electrons are not stable enough in solutions, the complex ion is capable of conducting electricity.
HCO3- is regulatory substance in human body, which help to balance acidity in Kidneys. The complex structure of Bicarbonate after bonding with sodium, potassium and chlorides it becomes an electrolyte and regulates pH balance in body.
HCO3- a polyatomic ion
Polyatomic ions have more than two different atoms in its geometry. Different physical properties of different elements refer to polyatomic structure of the compounds.
HCO3- is a polyatomic ion as it is containing three Oxygen atoms, one carbon and one Hydrogen atom. Hydrocarbonate contains carbon oxoanion, which is the result of removal of proton from carbonic acid.
HCO3- a precipitate
Hydrocabonate is not a precipitate itself but it has the property of giving precipitation of some compounds after added with metals like Sodium, Potassium and few more.

When NaCl is added with NH4HCO3, it gives out a precipitation of NaHCO3 that is Sodium hydrocarbonate. Here the ion shows its ability in forming precipitate.
We can conclude that the Lewis structure of bicarbonate ion (HCO3-) is faithful to reveal internal facts regarding electronic arrangement of the ion. The VSEPR theory has been evaluated to identify the shape and angle of the compound through describing lone pair and bond pair structure of the HCO3-. I have implemented both physical and chemical both kind of properties of Bicarbonate ion in this article.
- Lewis Structures: Facts You Must Know
- H2SO4 + NaOH: A Powerful Chemical Reaction Unveiled
- Valence Electrons: The Key to Understanding Chemical Reactions
- Sodium Carbonate: Understanding Its Uses, Benefits, and Safety
- Electronegativity Uncovered: A Deep Dive into Chemical Bonding
- Electronegativity Unveiled: Understanding Its Impact on Chemical Bonds
Also Read:



![7 Facts On Cu[(nh3)4] 2+ Lewis Structure, Characteristics 14 download 2 1](https://lambdageeks.com/wp-content/uploads/2023/12/download-2-1.png)
![7 Facts On Cu[(nh3)4] 2+ Lewis Structure, Characteristics 15 cu[(nh3)4] 2+ lewis structure](https://lambdageeks.com/wp-content/uploads/2022/06/download-2.png)
![7 Facts On Cu[(nh3)4] 2+ Lewis Structure, Characteristics 16 cu[(nh3)4] 2+ lewis structure](https://lambdageeks.com/wp-content/uploads/2022/06/images-1.jpg)