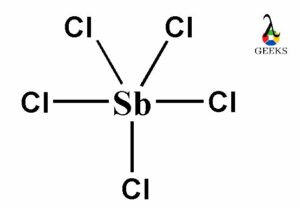
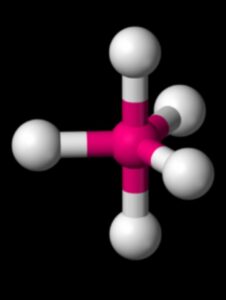
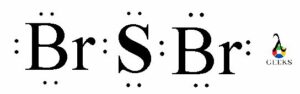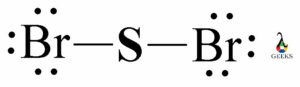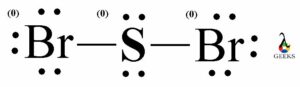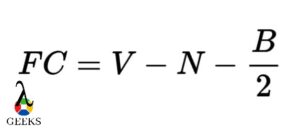The PF5 Lewis structure refers to the arrangement of atoms and electrons in a molecule of phosphorus pentafluoride (PF5). In this structure, phosphorus is the central atom bonded to five fluorine atoms. The Lewis structure helps us understand the bonding and electron distribution within the molecule. It is represented by drawing the symbol for phosphorus in the center, surrounded by the symbols for fluorine atoms, with lines representing the bonds between them. The PF5 molecule has a trigonal bipyramidal shape, with three fluorine atoms in equatorial positions and two in axial positions. This arrangement allows for the optimal distribution of electrons.
Key Takeaways
| Lewis Structure | Molecular Shape |
|---|---|
| PF5 | Trigonal Bipyramidal |
Understanding Lewis Structures
What is a Lewis Structure?
A Lewis structure is a diagram that represents the arrangement of atoms and valence electrons in a molecule. It was developed by Gilbert N. Lewis in 1916 as a way to visualize chemical bonding. Lewis structures are also known as Lewis dot diagrams or electron dot structures.
In a Lewis structure, the valence electrons of an atom are represented by dots or lines. Each dot represents one valence electron, while each line represents a covalent bond. The goal of drawing a Lewis structure is to satisfy the octet rule, which states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with eight valence electrons.
How to Draw a Lewis Structure
Drawing a Lewis structure involves several steps. Let’s take the example of Phosphorus pentafluoride (PF5) to understand the process.
-
Determine the total number of valence electrons in the molecule. For PF5, phosphorus (P) is in Group 5A and has 5 valence electrons, while each fluorine (F) atom has 7 valence electrons. Therefore, the total number of valence electrons in PF5 is 5 + (5 × 7) = 40.
-
Identify the central atom. In PF5, phosphorus is the central atom as it is less electronegative than fluorine.
-
Place the central atom in the center and connect it to the surrounding atoms with single bonds. In PF5, phosphorus is bonded to each fluorine atom.
-
Distribute the remaining electrons around the atoms to satisfy the octet rule. Start by placing lone pairs on the outer atoms (fluorine) and then distribute the remaining electrons on the central atom (phosphorus). Remember that each bond consists of two electrons.
-
Check if all atoms have achieved an octet or a stable electron configuration. In PF5, phosphorus has 10 valence electrons (5 bonds and 2 lone pairs), while each fluorine atom has 8 valence electrons (1 bond and 3 lone pairs). The total number of valence electrons is still 40.
-
If necessary, use double or triple bonds to satisfy the octet rule. In PF5, phosphorus can form a double bond with one of the fluorine atoms to achieve an octet.
By following these steps, we can draw the Lewis structure of PF5, which consists of a central phosphorus atom bonded to five fluorine atoms. The structure has one double bond and four single bonds, with each atom achieving an octet.
Lewis structures are useful in understanding the chemical bonding and molecular geometry of a compound. They provide insights into the electron pair geometry and molecular shape of a molecule. In the case of PF5, the electron pair geometry is trigonal bipyramidal, and the molecular shape is also trigonal bipyramidal.
It is important to note that Lewis structures are a simplified representation of molecules and do not account for the three-dimensional nature of molecules. To understand the actual shape of a molecule, we use the VSEPR (Valence Shell Electron Pair Repulsion) theory, which takes into account the repulsion between electron pairs to predict molecular shapes.
The Lewis Structure of PF5
How to Draw the Lewis Structure for PF5

To understand the Lewis structure of PF5 (Phosphorus pentafluoride), we need to consider the valence electrons in PF5 and the octet rule. Phosphorus (P) is in Group 5A, so it has five valence electrons. Fluorine (F) is in Group 7A, so it has seven valence electrons. In PF5, there is one phosphorus atom bonded to five fluorine atoms.
To draw the Lewis structure for PF5, follow these steps:
-
Determine the total number of valence electrons: Phosphorus contributes 5 valence electrons, and each fluorine contributes 7 valence electrons. So, the total number of valence electrons in PF5 is 5 + (5 * 7) = 40.
-
Place the atoms in the structure: Phosphorus is the central atom, and the fluorine atoms are bonded to it. Since fluorine is more electronegative than phosphorus, it is more likely to be found on the outside of the structure.
-
Connect the atoms with single bonds: Each fluorine atom forms a single bond with the phosphorus atom. This accounts for 5 of the valence electrons.
-
Distribute the remaining electrons: After accounting for the single bonds, there are 35 valence electrons left. Place them as lone pairs around the fluorine atoms, ensuring that each atom has an octet (except for phosphorus).
-
Check for octets and adjust if necessary: Phosphorus does not have an octet yet. To complete its octet, move a lone pair from one of the fluorine atoms to form a double bond with phosphorus. This gives phosphorus a total of 8 valence electrons.
The final Lewis structure for PF5 is as follows:
F
/
F -- P -- F
F
Identifying the Correct Lewis Structure for PF5
To identify the correct Lewis structure for PF5, we need to consider the octet rule and the electron pair geometry. In PF5, the phosphorus atom is surrounded by five fluorine atoms, resulting in a trigonal bipyramidal electron pair geometry.
The molecular shape of PF5 is also trigonal bipyramidal, with the phosphorus atom at the center and the fluorine atoms positioned around it. The bond angle between the phosphorus atom and the fluorine atoms is approximately 90 degrees.
Understanding the PF5 Lewis Dot Structure
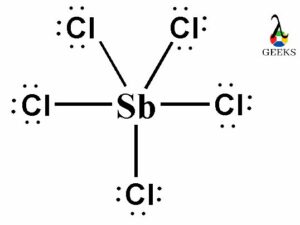
In the Lewis dot structure of PF5, the phosphorus atom is represented by the letter P, and the fluorine atoms are represented by the letter F. The lines between the atoms represent covalent bonds, where electrons are shared between the atoms.
The Lewis dot structure of PF5 shows that there are no lone pairs of electrons on the phosphorus atom. All the valence electrons are either involved in bonding or shared between the atoms.
It is important to note that the PF5 molecule is nonpolar due to the symmetrical arrangement of the fluorine atoms around the central phosphorus atom. This means that the molecule does not have a positive or negative end, resulting in a balanced distribution of charge.
Detailed Analysis of PF5 Lewis Structure
Phosphorus pentafluoride (PF5) is a chemical compound composed of one phosphorus atom bonded to five fluorine atoms. To understand the structure of PF5, we can analyze its Lewis structure, which represents the arrangement of atoms and valence electrons in a molecule.
PF5 Lewis Structure Shape

The shape of a molecule is determined by its electron pair geometry and molecular geometry. In the case of PF5, the electron pair geometry is trigonal bipyramidal, while the molecular geometry is also trigonal bipyramidal. This means that the five fluorine atoms are arranged around the central phosphorus atom in a trigonal bipyramidal shape.
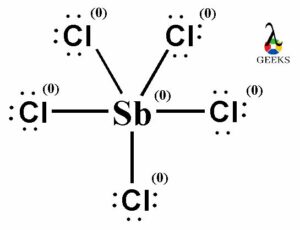
PF5 Lewis Structure Formal Charges
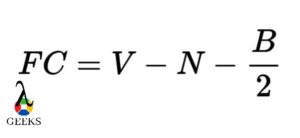
Formal charges are used to determine the distribution of electrons in a molecule. In the Lewis structure of PF5, each fluorine atom is bonded to the phosphorus atom, resulting in a formal charge of zero for each atom. The phosphorus atom also has a formal charge of zero. This distribution of formal charges ensures that the molecule is stable.
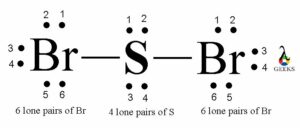
Number of Lone Pairs in PF5 Lewis Structure

Lone pairs are pairs of electrons that are not involved in bonding. In the Lewis structure of PF5, there are no lone pairs present on the central phosphorus atom. All the valence electrons of phosphorus are involved in bonding with the fluorine atoms. Therefore, PF5 does not have any lone pairs.
PF5 Lewis Structure Octet Rule

The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with eight valence electrons. In the case of PF5, the phosphorus atom has five valence electrons, and each fluorine atom has seven valence electrons. By forming covalent bonds, the phosphorus atom shares its five valence electrons with the five fluorine atoms, resulting in a complete octet for each atom.
PF5 Lewis Structure Resonance
Resonance structures are different representations of a molecule that can be drawn by moving electrons within the molecule. However, in the case of PF5, resonance structures are not applicable as there are no multiple bonding possibilities or delocalized electrons.
PF5 Lewis Structure Bond Angle
The bond angle in PF5 refers to the angle between the phosphorus atom and the fluorine atoms. In a trigonal bipyramidal geometry, the bond angle between the equatorial fluorine atoms is 120 degrees, while the bond angle between the axial fluorine atoms and the phosphorus atom is 90 degrees. These bond angles contribute to the overall shape of the PF5 molecule.
PF5 Lewis Structure Electron Geometry
The electron geometry of PF5 is trigonal bipyramidal, as mentioned earlier. This geometry takes into account both the bonded atoms and the lone pairs, if present. In the case of PF5, there are no lone pairs, so the electron geometry is the same as the molecular geometry.
PF5 Lewis Structure Molecular Geometry
The molecular geometry of PF5 is also trigonal bipyramidal, as mentioned earlier. This geometry describes the arrangement of only the bonded atoms, excluding any lone pairs. The five fluorine atoms are symmetrically arranged around the central phosphorus atom, resulting in a trigonal bipyramidal shape for the PF5 molecule.
Hybridization in PF5
Understanding Hybridization
In order to understand hybridization in PF5 (phosphorus pentafluoride), let’s first discuss the concept of hybridization. Hybridization is a phenomenon in which atomic orbitals mix to form new hybrid orbitals. These hybrid orbitals have different shapes and energies compared to the original atomic orbitals. Hybridization occurs when an atom forms covalent bonds with other atoms.
In the case of PF5, phosphorus (P) is the central atom surrounded by five fluorine (F) atoms. To determine the hybridization of the central atom, we need to consider the valence electrons in PF5. Phosphorus has five valence electrons, and each fluorine atom contributes one valence electron, resulting in a total of ten valence electrons.
According to the octet rule, atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight valence electrons. In the case of PF5, phosphorus can achieve an octet by forming five covalent bonds with fluorine atoms.
Hybridization in Pentafluorophosphorane

To understand the hybridization in pentafluorophosphorane (PF5) in more detail, let’s analyze the molecular structure and bonding.
The Lewis dot diagram of PF5 shows that phosphorus is surrounded by five fluorine atoms, each sharing a single bond with the central phosphorus atom. This suggests that the phosphorus atom in PF5 is sp3d hybridized.
In sp3d hybridization, one s orbital, three p orbitals, and one d orbital from the phosphorus atom combine to form five sp3d hybrid orbitals. These hybrid orbitals are directed towards the five fluorine atoms, resulting in a trigonal bipyramidal electron pair geometry.
The molecular shape of PF5 is also trigonal bipyramidal, with the five fluorine atoms positioned around the central phosphorus atom. The bond angle between the phosphorus atom and the fluorine atoms is approximately 90 degrees.
In terms of chemical bonding, the phosphorus atom forms covalent bonds with the fluorine atoms by sharing electron pairs. The electronegativity difference between phosphorus and fluorine is significant, resulting in polar covalent bonds. However, due to the symmetric arrangement of the fluorine atoms, the molecule as a whole is nonpolar.
It is important to note that PF5 can exhibit resonance structures due to the presence of lone pairs on the phosphorus atom. These resonance structures contribute to the overall stability of the molecule.
Polarity of PF5
Understanding Polarity
When it comes to understanding the polarity of molecules, it’s important to consider the arrangement of atoms and the distribution of electrons. In the case of PF5 (phosphorus pentafluoride), we can explore its polarity by examining its Lewis structure, molecular geometry, and the presence of any lone pairs.
Is PF5 Polar or Nonpolar?
To determine whether PF5 is polar or nonpolar, we need to analyze its molecular geometry. The Lewis dot diagram for PF5 shows that phosphorus (P) is surrounded by five fluorine (F) atoms. This molecule has a trigonal bipyramidal electron pair geometry, with the phosphorus atom at the center and the fluorine atoms positioned around it.
According to the VSEPR (Valence Shell Electron Pair Repulsion) theory, the electron pairs in PF5 will arrange themselves in a way that minimizes repulsion. In this case, the three fluorine atoms are positioned in an equatorial plane, while the other two fluorine atoms are in axial positions. The bond angles between the phosphorus atom and the fluorine atoms are approximately 90 degrees for the axial positions and 120 degrees for the equatorial positions.
PF5 Lewis Structure: Polar or Nonpolar?
To determine the polarity of PF5, we need to consider the presence of any lone pairs on the central phosphorus atom. In the case of PF5, there are no lone pairs on the phosphorus atom. This means that all the electron pairs are involved in bonding with the fluorine atoms.
Since PF5 has a symmetrical arrangement of atoms and no lone pairs, the individual bond polarities cancel each other out. As a result, PF5 is a nonpolar molecule. The electronegativity difference between phosphorus and fluorine is not significant enough to create a dipole moment, leading to a nonpolar molecule.
By understanding the phosphorus pentafluoride structure and its molecular geometry, we can determine that PF5 is a nonpolar molecule. This knowledge of PF5’s polarity is essential in understanding its chemical bonding and behavior in various reactions.
Properties and Uses of PF5
Phosphorus pentafluoride (PF5) is a chemical compound that exhibits interesting properties and finds various practical applications. Let’s explore some key aspects of PF5, including its stability, molecular nature, and uses.
Is PF5 Stable?
PF5 is a stable compound under normal conditions. It is a solid at room temperature and pressure, appearing as a white crystalline powder. However, it is important to handle PF5 with care as it can react vigorously with water and other reactive substances.
Is PF5 a Molecular Compound?
Yes, PF5 is a molecular compound. It consists of a central phosphorus atom bonded to five fluorine atoms. The Lewis dot diagram for PF5 shows that phosphorus contributes one electron, while each fluorine atom contributes one electron, resulting in a total of 40 valence electrons. These electrons are involved in the formation of covalent bonds between phosphorus and fluorine.
Is PF5 Ionic?
No, PF5 is not an ionic compound. Ionic compounds typically involve the transfer of electrons between atoms, resulting in the formation of positive and negative ions. In the case of PF5, the sharing of electrons between phosphorus and fluorine atoms forms covalent bonds, where electrons are shared rather than transferred.
Practical Uses of PF5
PF5 has several practical applications in various industries. Here are some notable uses of PF5:
-
Fluorination Reactions: PF5 is commonly used as a fluorinating agent in organic synthesis. It can introduce fluorine atoms into organic molecules, leading to the formation of new compounds with desired properties. This is particularly useful in pharmaceutical and agrochemical industries.
-
Etching Agent: PF5 is utilized in the semiconductor industry as an etching agent. It can selectively remove certain materials from the surface of semiconductors, allowing for precise patterning and fabrication of electronic devices.
-
Catalyst: PF5 can act as a catalyst in various chemical reactions. It can enhance the rate of certain reactions without being consumed in the process. This property makes it valuable in industrial processes where increased reaction rates are desired.
-
Solvent: PF5 can serve as a solvent for certain reactions and processes. Its unique properties make it suitable for dissolving and stabilizing specific compounds, enabling efficient chemical transformations.
References
In chemistry, references are an essential part of understanding and verifying scientific information. They provide a way to access the sources that have been used to support the claims and findings presented in a particular study or article. By referring to these sources, readers can delve deeper into the subject matter and gain a more comprehensive understanding of the topic at hand.
When studying the structure and properties of phosphorus pentafluoride (PF5), several key concepts come into play. Understanding the Lewis dot diagram, molecular geometry, valence electrons, chemical bonding, octet rule, electron pair geometry, molecular shape, covalent bonds, and the bonding between phosphorus and fluorine are crucial to comprehending the behavior and characteristics of PF5.
To visualize the structure of PF5, we can draw a Lewis dot diagram. Phosphorus, with its atomic symbol P, is located at the center, while the five fluorine atoms, represented by the symbol F, are positioned around it. Each fluorine atom shares a covalent bond with the central phosphorus atom, resulting in a molecule with a trigonal bipyramidal shape. This arrangement allows for the optimal distribution of electron pairs and minimizes repulsion between them.
In terms of polarity, PF5 is a nonpolar molecule. This is due to the symmetrical arrangement of the fluorine atoms around the central phosphorus atom, resulting in a cancellation of dipole moments. Although the individual phosphorus-fluorine bonds are polar, the overall molecule does not possess a net dipole moment.
The VSEPR (Valence Shell Electron Pair Repulsion) theory helps us determine the molecular shape of PF5. According to this theory, the five electron pairs around the central phosphorus atom arrange themselves in a way that minimizes repulsion. Three of these electron pairs are bonding pairs, while the remaining two are lone pairs. The AXE notation, which stands for “A” representing the central atom, “X” representing the surrounding atoms, and “E” representing the lone pairs, can be used to describe the molecular geometry of PF5 as AX3E2.
Frequently Asked Questions
What is the Lewis structure of PF5?
The Lewis structure of Phosphorus Pentafluoride (PF5) consists of the central atom Phosphorus (P) bonded to five Fluorine atoms (F). Phosphorus has 5 valence electrons and each Fluorine atom has 7 valence electrons, making a total of 40 valence electrons in the PF5 molecule. The Lewis structure shows that all the atoms in the molecule have achieved an octet configuration.
How does the molecular geometry of PF5 look like?
The molecular geometry of PF5 is trigonal bipyramidal. This is due to the five bonding pairs of electrons around the central Phosphorus atom. The VSEPR theory predicts this shape because it minimizes the repulsion forces between the electron pairs.
What is the valence electron configuration in PF5?
The valence electron configuration in PF5 is 5 for Phosphorus and 7 for each Fluorine atom. This totals to 40 valence electrons for the entire molecule.
How does hybridization occur in the PF5 structure?
In the PF5 structure, hybridization occurs in the central Phosphorus atom. It undergoes sp3d hybridization, which is the combination of one s, three p, and one d orbital. This results in five hybrid orbitals, aligning themselves in a trigonal bipyramidal shape.
What is the formal charge in the PF5 Lewis structure?
The formal charge in the PF5 Lewis structure is zero. This is because the total number of valence electrons and the total number of electrons assigned to each atom in the molecule are equal.
How does resonance occur in the PF5 structure?
Resonance does not occur in the PF5 structure. This is because all the P-F bonds are equivalent and there are no lone pairs of electrons on the central Phosphorus atom that could lead to the formation of resonance structures.
How does the Lewis dot structure of PF5 look like?
The Lewis dot structure of PF5 shows the central Phosphorus atom surrounded by five Fluorine atoms. Each P-F bond is represented by a pair of dots, indicating the sharing of an electron pair between the Phosphorus and Fluorine atoms.
What is the bond angle in the PF5 structure?
The bond angle in the PF5 structure is 120 degrees in the equatorial plane and 90 degrees between the equatorial and axial positions. This is due to the trigonal bipyramidal molecular geometry of PF5.
Is PF5 a molecular compound?
Yes, PF5 is a molecular compound. It is composed of nonmetals, Phosphorus and Fluorine, and they share electrons to form covalent bonds.
How does the octet rule apply to the PF5 structure?
The octet rule applies to the PF5 structure in that all the atoms in the molecule achieve an octet configuration. The central Phosphorus atom exceeds the octet rule due to its ability to expand its valence shell and accommodate more than 8 electrons.
Also Read: