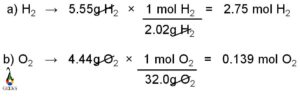
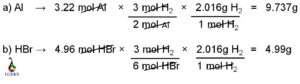
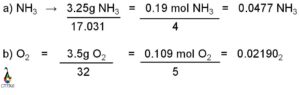
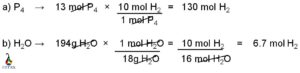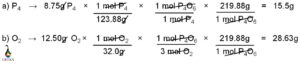Calculating the molar mass from the freezing point is a useful technique in chemistry. By measuring the freezing point depression caused by the addition of a solute to a solvent, we can determine the molar mass of the solute. This method is based on the principle that the freezing point of a solution is lower than that of the pure solvent due to the presence of solute particles. By quantifying the extent of this depression, we can calculate the molar mass of the solute using the equation ΔT = Kf * m, where ΔT is the freezing point depression, Kf is the cryoscopic constant, and m is the molality of the solution.
Key Takeaways
The following table provides a concise summary of the key information for calculating molar mass from freezing point:
| Equation | Description |
|---|---|
| ΔT = Kf * m | The equation used to calculate the freezing point depression |
| ΔT | Freezing point depression |
| Kf | Cryoscopic constant |
| m | Molality of the solution |
Please note that the table above is not exhaustive, but it highlights the essential components necessary for understanding the calculation of molar mass from freezing point.
Understanding Freezing Point Depression
Definition and Explanation
Freezing point depression is a phenomenon that occurs when the freezing point of a solvent is lowered by the presence of a solute. This is a fundamental concept in solution chemistry and is classified as a colligative property. Colligative properties depend on the number of solute particles present in a solution, rather than the specific nature of the solute itself.
To understand freezing point depression, we need to consider the concept of molality. Molality is a measure of the concentration of a solute in a solvent and is defined as the number of moles of solute per kilogram of solvent. It is denoted by the symbol ‘m’.
The freezing point depression can be calculated using the equation:
δTf = Kf * m
Where:
– δTf is the depression in freezing point
– Kf is the molal freezing point depression constant, which is specific to each solvent
– m is the molality of the solution
The value of Kf can be experimentally determined for different solvents and is a measure of the solvent’s properties. It is important to note that the freezing point depression is directly proportional to the molality of the solution.
How Molar Mass Affects Freezing Point
The molar mass of a solute plays a crucial role in determining the extent of freezing point depression. The molar mass is the mass of one mole of a substance and is expressed in atomic mass units (amu). It can be calculated by summing the atomic masses of all the atoms in the chemical formula of the compound.
When a solute is added to a solvent, the solute-solvent interaction disrupts the regular arrangement of solvent molecules during the phase transition from liquid to solid. This disruption leads to a decrease in the freezing point of the solvent.
The relationship between molar mass and freezing point depression can be explained using the Van’t Hoff factor (i). The Van’t Hoff factor accounts for the number of particles into which a solute dissociates in a solution. For non-electrolytes, the Van’t Hoff factor is 1, as they do not dissociate into ions. However, for electrolytes, the Van’t Hoff factor is greater than 1, as they dissociate into ions.
The equation for calculating the freezing point depression taking into account the Van’t Hoff factor is:
δTf = i * Kf * m
From this equation, we can see that the molar mass indirectly affects the freezing point depression through the Van’t Hoff factor. A higher molar mass generally leads to a lower Van’t Hoff factor, resulting in a smaller freezing point depression.
To determine the molar mass of a solute from the freezing point depression, we can rearrange the equation as follows:
molar mass = (δTf / (Kf * i)) * 1000 / molality
By substituting the known values of freezing point depression (δTf), molal freezing point depression constant (Kf), Van’t Hoff factor (i), and molality into the equation, we can calculate the molar mass of the solute.
The Process of Calculating Molar Mass from Freezing Point
Necessary Tools and Materials
To calculate the molar mass from freezing point, you will need the following tools and materials:
- Naphthalene: This is the solute that will be used in the experiment. Naphthalene is a common compound that can be easily obtained.
- Water: This will be the solvent in which the naphthalene is dissolved. Water is a widely available and easily accessible solvent.
- Thermometer: A thermometer is necessary to measure the freezing point of the solution accurately.
- Cryoscopic Constant (Kf): This constant is specific to each solvent and is used in the equation to determine the molar mass.
- Balance: A balance is required to measure the mass of the naphthalene accurately.
- Beaker: A beaker is needed to hold the water and naphthalene solution during the experiment.
Step-by-Step Guide
Now that you have all the necessary tools and materials, let’s dive into the step-by-step guide on how to calculate the molar mass from freezing point:
- Measure the mass of an empty beaker using the balance. Record this mass as the initial mass.
- Add a known mass of naphthalene to the beaker and measure the total mass. Record this mass as the final mass.
- Calculate the mass of naphthalene by subtracting the initial mass from the final mass.
- Measure a known volume of water using a graduated cylinder and pour it into the beaker containing the naphthalene.
- Stir the solution gently to ensure that the naphthalene is completely dissolved in the water.
- Place the beaker with the solution on a heat source and start heating it slowly.
- Continuously monitor the temperature of the solution using the thermometer.
- As the temperature decreases, you will observe a point where the solution starts to freeze. This is the freezing point.
- Record the freezing point of the solution in degrees Celsius.
- Use the equation δTf = Kf * molality to calculate the molality of the solution. Here, δTf represents the depression in freezing point, Kf is the molal freezing point depression constant specific to water, and molality is the concentration of the solute in moles per kilogram of solvent.
- Calculate the number of moles of naphthalene by multiplying the molality by the mass of the solvent in kilograms.
- Determine the molar mass of naphthalene by dividing the mass of naphthalene by the number of moles.
- Congratulations! You have successfully calculated the molar mass of naphthalene using the freezing point depression method.
By following this step-by-step guide, you can determine the molar mass of different chemical compounds using the freezing point depression method. This process utilizes the principles of colligative properties, solute-solvent interaction, and the mole concept in solution chemistry. It is an essential technique in physical chemistry and provides valuable insights into the molecular properties of substances.
Practical Examples
Calculating Molar Mass of an Unknown Compound Dissolved in Water
In this practical example, we will explore how to calculate the molar mass of an unknown compound dissolved in water. This is an important concept in chemistry as it helps us determine the molecular weight of a substance, which is crucial for various calculations and understanding its properties.
To calculate the molar mass, we need to consider the freezing point depression caused by the presence of the unknown compound in water. Freezing point depression is one of the colligative properties, which depend on the number of solute particles present in a solution.
To determine the molar mass, we can use the equation:
δTf = Kf * molality
Where:
– δTf is the depression in freezing point
– Kf is the molal freezing point depression constant for water
– molality is the concentration of the solute in moles per kilogram of solvent
By measuring the freezing point depression and knowing the Kf value for water, we can calculate the molality of the solution. From there, using the stoichiometric coefficients from the chemical formula of the unknown compound, we can determine the molar mass.
Determining Molar Mass of a Certain Sugar Dissolved in Water
In this example, we will focus on determining the molar mass of a specific sugar dissolved in water. Sugars are organic compounds that are commonly found in various foods and beverages. Understanding their molar mass is essential for various applications, such as determining their sweetness or conducting chemical reactions involving sugars.
To determine the molar mass of the sugar, we can follow a similar approach as in the previous example. By measuring the freezing point depression caused by the sugar in water and knowing the Kf value for water, we can calculate the molality of the solution. From there, using the stoichiometric coefficients from the molecular formula of the sugar, we can determine its molar mass.
Calculating Molar Mass of an Unknown Nonelectrolyte Compound Dissolved in Benzene
Moving on to a different solvent, let’s consider the calculation of the molar mass of an unknown nonelectrolyte compound dissolved in benzene. Benzene is an organic solvent commonly used in various chemical reactions and processes.
Similar to the previous examples, we can determine the molar mass by measuring the freezing point depression caused by the unknown compound in benzene and knowing the Kf value for benzene. By calculating the molality of the solution and considering the stoichiometric coefficients from the chemical formula of the unknown compound, we can determine its molar mass.
Determining Molar Mass of Naphthalene Added to Benzene
In this example, we will focus on determining the molar mass of naphthalene added to benzene. Naphthalene is a common aromatic hydrocarbon that is often used as a moth repellent and in the production of dyes and resins.
To determine the molar mass of naphthalene, we can again utilize the freezing point depression method. By measuring the freezing point depression caused by naphthalene in benzene and knowing the Kf value for benzene, we can calculate the molality of the solution. From there, considering the stoichiometric coefficients from the molecular formula of naphthalene, we can determine its molar mass.
These practical examples demonstrate the application of molar mass calculation and freezing point depression in determining the molecular weight of various compounds dissolved in different solvents. By understanding the solute-solvent interaction, utilizing the cryoscopic constant, and considering the molality and solvent properties, we can accurately determine the molar mass of chemical compounds.
Common Mistakes and Troubleshooting
Common Errors in Calculating Molar Mass from Freezing Point
When it comes to calculating the molar mass from freezing point depression, there are a few common mistakes that can trip you up. Let’s take a look at some of these errors and how to avoid them.
-
Incorrect use of the freezing point depression equation: One common mistake is not using the correct equation for calculating freezing point depression. The equation, ΔTf = Kf * m, relates the change in freezing point (ΔTf) to the molal concentration (m) and the molal freezing point depression constant (Kf). Make sure to use this equation correctly to obtain accurate results.
-
Failure to consider the Van’t Hoff factor: The Van’t Hoff factor (i) takes into account the number of particles a solute dissociates into in a solution. For example, if a solute dissociates into two particles, the Van’t Hoff factor would be 2. Failing to consider this factor can lead to incorrect molar mass calculations. Be sure to include the appropriate Van’t Hoff factor in your calculations.
-
Using the wrong freezing point depression constant: Different solvents have different molal freezing point depression constants (Kf). Using the wrong Kf value can result in inaccurate calculations. Always double-check that you are using the correct Kf value for the solvent you are working with.
-
Inaccurate determination of freezing point: Precise determination of the freezing point is crucial for accurate molar mass calculations. Errors in measuring the freezing point can lead to significant deviations in the calculated molar mass. Make sure to use reliable and calibrated equipment to determine the freezing point of the solution.
Tips for Accurate Calculations
To ensure accurate calculations when determining molar mass from freezing point depression, consider the following tips:
-
Use precise measurements: Accurate measurements of the freezing point and the mass of the solute are essential for reliable calculations. Use calibrated instruments and take multiple measurements to minimize errors.
-
Consider the solute-solvent interaction: The nature of the solute-solvent interaction can affect the freezing point depression. Consider the properties of the solvent and how it interacts with the solute when performing calculations.
-
Pay attention to the chemical formula: Ensure that you have the correct chemical formula for the solute. Mistakes in the chemical formula can lead to incorrect molar mass calculations.
-
Take into account stoichiometric coefficients: If the chemical equation for the solute involves stoichiometric coefficients, make sure to consider them when calculating the molar mass. The stoichiometric coefficients indicate the ratio of moles of each component in the reaction.
Remember, accurate calculations of molar mass from freezing point depression require attention to detail and careful consideration of various factors. By avoiding common errors and following these tips, you can improve the accuracy of your calculations in solution chemistry.
Advanced Concepts
In the field of chemistry, there are several advanced concepts that are crucial for understanding various phenomena and solving complex problems. Two such concepts are “Calculating Freezing Point from Molarity” and “Calculating Molar Mass from Percent Composition“. Let’s explore these concepts in detail.
Calculating Freezing Point from Molarity
When a solute is dissolved in a solvent, it affects the freezing point of the resulting solution. This phenomenon is known as freezing point depression, which is one of the colligative properties of solutions. The extent of freezing point depression depends on the concentration of the solute in the solution, which is often expressed in terms of molarity.
To calculate the freezing point depression, we can use the equation:
δTf = Kf * m
Where:
– δTf is the depression in freezing point
– Kf is the molal freezing point depression constant for the solvent
– m is the molality of the solution (moles of solute per kilogram of solvent)
By knowing the Kf value for a specific solvent and the molality of the solution, we can determine the freezing point depression and subsequently the freezing point of the solution.
Calculating Molar Mass from Percent Composition
The molar mass of a compound is the mass of one mole of that compound and is an essential parameter in various calculations in chemistry. One way to determine the molar mass is by using the percent composition of the compound.
The percent composition of a compound provides the relative masses of each element present in the compound. To calculate the molar mass from percent composition, we follow these steps:
- Convert the percent composition of each element into grams.
- Divide the mass of each element by its atomic mass in atomic mass units (amu).
- Determine the stoichiometric coefficients of each element in the chemical formula.
- Multiply the number of moles of each element by its stoichiometric coefficient.
- Sum up the moles of all elements to obtain the molar mass of the compound.
By considering the percent composition and using the mole concept, we can calculate the molar mass of a compound accurately.
These advanced concepts in chemistry, namely “Calculating Freezing Point from Molarity” and “Calculating Molar Mass from Percent Composition,” are fundamental in understanding the thermodynamics and solution chemistry. They provide valuable insights into the solute-solvent interaction, phase transitions, and the determination of freezing points and molar masses of chemical compounds.
Remember to consider the relevant constants, such as the cryoscopic constant (Kf), and the properties of the solvent when applying these equations and concepts.
Frequently Asked Questions
1. How to find molecular weight from freezing point depression?

The molecular weight of a substance can be determined from the freezing point depression using the formula ΔT = Kf * m, where ΔT is the freezing point depression, Kf is the cryoscopic constant of the solvent, and m is the molality of the solution. The molecular weight can then be calculated by rearranging the formula to find m and then using the definition of molality (moles of solute/kg of solvent) to find the molecular weight.
2. How to calculate freezing point from molarity?
The freezing point of a solution can be calculated from its molarity using the formula ΔT = Kf * m, where ΔT is the freezing point depression, Kf is the cryoscopic constant of the solvent, and m is the molality of the solution. Note that molarity and molality are different, but can be converted to each other if the density of the solution is known.
3. Does molar mass affect freezing point?
Yes, the molar mass of a solute does affect the freezing point of a solution. The larger the molar mass, the fewer moles of solute are present in a given mass, resulting in a smaller freezing point depression.
4. How to get molar mass from freezing point depression?
The molar mass of a substance can be determined from the freezing point depression using the formula ΔT = Kf * m, where ΔT is the freezing point depression, Kf is the cryoscopic constant of the solvent, and m is the molality of the solution. The molar mass can then be calculated by rearranging the formula to find m and then using the definition of molality (moles of solute/kg of solvent) to find the molar mass.
5. How to determine molar mass from freezing point depression?
The molar mass of a substance can be determined from the freezing point depression using the formula ΔT = Kf * m, where ΔT is the freezing point depression, Kf is the cryoscopic constant of the solvent, and m is the molality of the solution. The molar mass can then be calculated by rearranging the formula to find m and then using the definition of molality (moles of solute/kg of solvent) to find the molar mass.
6. How to calculate minimum molar mass?
The minimum molar mass can be calculated by using the freezing point depression and the Van’t Hoff factor. The formula is ΔT = Kf * i * m, where ΔT is the freezing point depression, Kf is the cryoscopic constant, i is the Van’t Hoff factor, and m is the molality. By rearranging the formula to solve for m and then using the definition of molality (moles of solute/kg of solvent), you can find the minimum molar mass.
7. How to calculate molar mass from freezing point?

The molar mass of a substance can be determined from the freezing point using the formula ΔT = Kf * m, where ΔT is the freezing point depression, Kf is the cryoscopic constant of the solvent, and m is the molality of the solution. The molar mass can then be calculated by rearranging the formula to find m and then using the definition of molality (moles of solute/kg of solvent) to find the molar mass.
8. How to calculate molecular weight from freezing point depression?
The molecular weight of a substance can be determined from the freezing point depression using the formula ΔT = Kf * m, where ΔT is the freezing point depression, Kf is the cryoscopic constant of the solvent, and m is the molality of the solution. The molecular weight can then be calculated by rearranging the formula to find m and then using the definition of molality (moles of solute/kg of solvent) to find the molecular weight.
9. How to calculate molar mass using freezing point depression?
The molar mass of a substance can be determined from the freezing point depression using the formula ΔT = Kf * m, where ΔT is the freezing point depression, Kf is the cryoscopic constant of the solvent, and m is the molality of the solution. The molar mass can then be calculated by rearranging the formula to find m and then using the definition of molality (moles of solute/kg of solvent) to find the molar mass.
10. How to calculate molar mass from freezing point depression?
The molar mass of a substance can be determined from the freezing point depression using the formula ΔT = Kf * m, where ΔT is the freezing point depression, Kf is the cryoscopic constant of the solvent, and m is the molality of the solution. The molar mass can then be calculated by rearranging the formula to find m and then using the definition of molality (moles of solute/kg of solvent) to find the molar mass.