In this article we find out O2 bond order and 7 facts regarding this.
The O2 molecule composed of 2 O atom. O atom has electronic configuration 1s22s22p4.Each O atom contains a total number of 8 electrons i.e. the whole O2 molecule contain a total number of 16 electrons. When atomic orbitals of O atom are mixed to form O2 molecule the energies of atomic orbitals changes i.e. some goes to higher energy level and some goes to lower energy level.
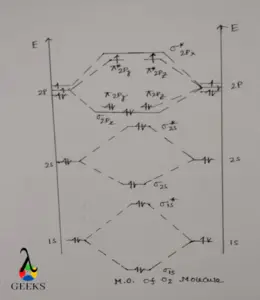
O2 bond order diagram
When 2 O atoms are engaged in forming O2 molecule, the atomic orbitals of O atom is also involved in the formation of O2 molecule. The total number of atomic orbital of O atom involved in forming O2 molecular diagram is 10 i.e. 5 atomic orbitals from each O atom.
These atomic orbitals are 1s,2s and 2p orbitals. 1s and 2s has only 1 orbit but 2p has 3 orbits. We see that there are a total of 10 atomic orbitals involved in O2 bond order diagram, hence molecular orbital also formed in the same number i.e. 10.
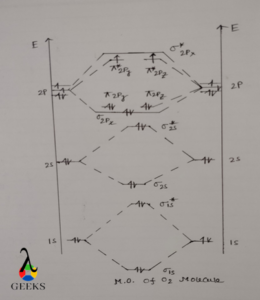
Molecular orbital diagram O2 bond order
At first, 1s orbital of both O atom are mixed to form 1 bonding MO i.e. σ1s and 1 antibonding MO i.e. σ1s* .Both molecular orbitals contains 2 electrons. σ1s bonding MO is lower in energy and σ1s* MO is higher in energy. Then 2s orbitals of each O atoms are coupled to form 1 σ2s bonding MO and 1 σ2s* antibonding MO. Similarly σ2s which is always lower in energy and σ2s* is always higher in energy.
.When 2p orbitals of each O atom are combined total 6 MO’s are formed, each 2p subshell contain 3 atomic orbitals i.e. 2px , 2py , 2pz . one 2px orbital of each O atom combined to form 1 σ2px , and 1 σ2px* MO. σ2px is bonding in nature and σ2px* MO is antibonding in nature. Out of these 2 MO only bonding σ2px MO is filled with 2 electrons but antibonding σ2px* MO remains vacant.
Then 2py and 2pz atomic orbitals of each O atom mixed together to form 4 MO’s. out of which 2 are ∏2py and ∏2pz and their corresponding antibonding MO’s i.e. ∏2py* and ∏2pz* . ∏2py and ∏2pz MO’s each contain 2 electrons making a total of 4 electrons in ∏ MO’s. ∏2py* and ∏2pz* each contain 1 electron making a total of 2 electrons in ∏*Mo’s.

Mot O2 bond order
The formula through which bond order is calculated is given below:
Bond Order= (Total no of electrons in bonding Mo-Total no of electrons in antibonding MO)/2 .
Total no of electrons in bonding MO of O2 molecule= 10.
Total no of electrons in antibonding MO of O2 molecule= 6.
Bond order of O2 molecule= 10-6/2= 2.
Hence one σ bond and 1 ∏ bond is formed between 2 O atom.
Bond order of O22-
When 2 electrons are added in O2 molecule O22- ion is formed. This is known as peroxide ion. These 2 electrons are added 1 electron each in higher energy ∏2py* and ∏2pz* MO’s. By the addition of these 2 electrons O22- ion contains a total of 18 electrons. These 18 electrons are filled similar way as in O2 with extra 2 electrons in ∏2py* and ∏2pz* MO’s.
The bond order of O22- ion is calculated by the above mentioned formula.
Total no of electrons in bonding MO of O22- ion=10.
Total no of electrons in antibonding MO of O22- ion=8.
Bond order of O22- ion= 10-8/2=1.
Hence there is only 1 bond which is σ bond is formed in O22- ion.
Covalent bond in O2 molecule
From O2 bond order diagram we see that there 2 covalent bonds between 2 O atoms. Hence 1 σ bond and 1 ∏ bond is formed between 2 O atoms. These 2 covalent bonds contains a total of 4 electrons.
The σ bond is formed by overlap of 2px orbital of each O atom and the ∏ bond is formed by the overlap of either 2py and 2pz orbitals of each O atom. The ∏ bond is higher in energy as well as weakly bonded than σ bond.
How to find O2 bond order?
To find out O2 bond order we can use the following formula,
Bond Order= (Total no of electrons in bonding MO-Total no of electrons in antibonding MO)/2 .
Total no of electrons in bonding MO of O2 molecule= 10.
Total no of electrons in antibonding MO of O2 molecule= 6.
Bond order of O2 molecule= 10-6/2= 2.
How many orbitals are singly occupied in O2?
When atomic orbitals of O atom are mixed to form O2 molecule the energies of atomic orbitals changes i.e. some goes to higher energy level and some goes to lower energy level. Higher energy orbitals are unstable in nature where as lower energy orbitals are stable. When we see the O2 bond order diagram, all the MO’s are fulfilled expect ∏2py* and ∏2pz* MO’s which are singly occupied.
Conclusion
From O2 bond order diagram we see that the bond order in O2 molecule is 2 i.e. 1 sigma bond and 1 pi bond is formed and in O22- ion the bond order is 1. Hence from bond order we see that O2 molecule is more stable than O22- ion.