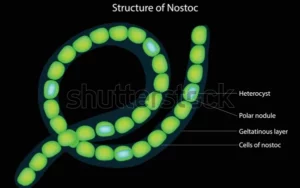
Prokaryotic cells, which include bacteria and archaea, do possess a cell wall, a rigid structure that lies outside the plasma membrane and provides additional protection to the cell. The cell wall is crucial for maintaining the cell’s shape and preventing osmotic lysis, which is the rupture of the cell due to the difference in osmotic pressure between the inside and outside of the cell.
The Composition of Prokaryotic Cell Walls
The chemical composition of the cell wall varies between different species of prokaryotes. Here’s a closer look at the cell wall composition of bacteria and archaea:
Bacterial Cell Walls
Bacterial cell walls contain peptidoglycan, a polymer made of sugars (N-acetylglucosamine and N-acetylmuramic acid) and amino acids (primarily L-alanine, D-glutamic acid, D-alanine, and diaminopimelic acid). Peptidoglycan is a unique component of bacterial cell walls and is not found in eukaryotic cells.
The peptidoglycan layer in bacterial cell walls can be thick or thin, depending on the species. Gram-positive bacteria have a thick peptidoglycan layer, while Gram-negative bacteria have a thinner peptidoglycan layer.
In addition to peptidoglycan, bacterial cell walls may also contain other molecules, such as:
- Teichoic acids: These are polymers of ribitol or glycerol phosphate found in the cell walls of Gram-positive bacteria.
- Lipoteichoic acids: These are amphiphilic molecules that anchor the teichoic acids to the cell membrane.
- Lipopolysaccharides (LPS): These are large molecules found in the outer membrane of Gram-negative bacteria, consisting of a lipid component (lipid A) and a polysaccharide component.
The composition and structure of the bacterial cell wall play a crucial role in determining the cell’s shape, rigidity, and resistance to environmental stresses.
Archaeal Cell Walls
Unlike bacterial cell walls, archaeal cell walls do not contain peptidoglycan. Instead, archaeal cell walls may contain a variety of other molecules, such as:
- Pseudopeptidoglycan: A peptidoglycan-like polymer found in some archaea, but with different chemical linkages.
- Glycoproteins: Proteins with covalently attached carbohydrate groups.
- Polysaccharides: Complex carbohydrates, such as glycans or heteropolysaccharides.
- Protein-based cell walls: Some archaea have cell walls composed primarily of proteins.
The diversity of archaeal cell wall compositions reflects the evolutionary adaptations of these organisms to various environmental conditions, such as extreme temperatures, pH, or salinity.
Gram Staining and Cell Wall Differences

Prokaryotic cells can be divided into two major groups based on their response to the Gram stain, a common laboratory technique used to differentiate bacteria:
-
Gram-positive bacteria: These bacteria have a thick cell wall composed of multiple layers of peptidoglycan, along with teichoic acids and lipoteichoic acids. The thick peptidoglycan layer gives Gram-positive bacteria a purple or blue color when stained with the Gram stain.
-
Gram-negative bacteria: These bacteria have a thinner peptidoglycan layer and an additional outer membrane containing lipopolysaccharides and lipoproteins. The thin peptidoglycan layer and the presence of the outer membrane give Gram-negative bacteria a red or pink color when stained with the Gram stain.
The differences in cell wall composition between Gram-positive and Gram-negative bacteria have important implications for their susceptibility to antibiotics, their interactions with the immune system, and their overall physiology.
The Importance of Prokaryotic Cell Walls
The cell wall is an essential structure for prokaryotic cells, serving several critical functions:
- Structural support: The cell wall provides structural integrity and maintains the shape of the cell, preventing it from bursting due to the high internal osmotic pressure.
- Protection: The cell wall acts as a barrier, protecting the cell from mechanical damage, osmotic stress, and environmental threats, such as antibiotics, toxins, and immune system components.
- Adhesion and interaction: The cell wall can facilitate the attachment of prokaryotic cells to surfaces, other cells, or host tissues, enabling them to colonize and interact with their environment.
- Cellular processes: The cell wall can play a role in various cellular processes, such as cell division, nutrient transport, and signal transduction.
The composition and properties of the prokaryotic cell wall can provide valuable information for identifying and classifying different species of bacteria and archaea, as well as for understanding their physiology, ecology, and interactions with their environment.
Conclusion
In summary, prokaryotic cells, including bacteria and archaea, do possess a cell wall, a crucial structural component that lies outside the plasma membrane. The cell wall composition varies between different species of prokaryotes, with bacteria typically having a peptidoglycan-based cell wall and archaea having a more diverse range of cell wall compositions. The differences in cell wall structure and composition have important implications for the physiology, ecology, and interactions of prokaryotic cells with their environment.
References:
- Lumen Learning. (n.d.). Structure of Prokaryotes. Retrieved from https://courses.lumenlearning.com/suny-biology2xmaster/chapter/structure-of-prokaryotes/
- Khan Academy. (n.d.). Prokaryote structure. Retrieved from https://www.khanacademy.org/science/ap-biology/gene-expression-and-regulation/dna-and-rna-structure/a/prokaryote-structure
- OpenStax. (n.d.). Unique Characteristics of Prokaryotic Cells. Retrieved from https://bio.libretexts.org/Bookshelves/Microbiology/Microbiology_%28OpenStax%29/03%3A_The_Cell/3.03%3A_Unique_Characteristics_of_Prokaryotic_Cells