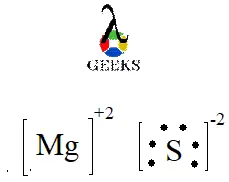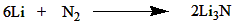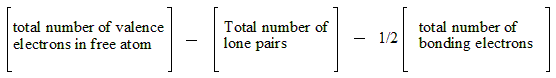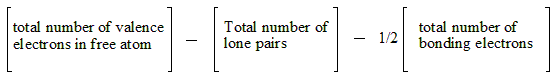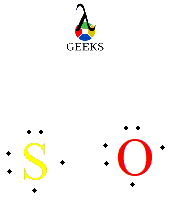The Lewis structure of MGS (Magnesium Sulfide) is a diagram that represents the arrangement of atoms and electrons in the molecule. It is a useful tool in understanding the chemical bonding and predicting the chemical properties of a compound. In the Lewis structure of MGS, the magnesium atom is represented by its symbol (Mg), and the sulfur atom is represented by its symbol (S). The electrons are shown as dots around the atoms, representing the valence electrons. The Lewis structure helps us determine the number of bonds and lone pairs of electrons in the molecule, which in turn gives us insights into its reactivity and stability.
Key Takeaways
| Atom | Symbol |
|---|---|
| Magnesium | Mg |
| Sulfur | S |
Understanding MGS Lewis Structure
The Lewis structure, also known as the Lewis dot structure, is a representation of the valence electrons in a chemical compound. It provides a visual depiction of the chemical bonding and molecular geometry of a molecule. In this article, we will explore the MGS Lewis structure and its various aspects, including how to draw it, the octet rule, resonance, lone pairs, formal charge, hybridization, and the shape of the molecule.
How to Draw MGS Lewis Structure

To draw the Lewis structure of MGS (Molecular Geometry Structure), we need to determine the total number of valence electrons present in the molecule. MGS is composed of three elements: M (Metal), G (Group), and S (Symbol). Each element contributes a certain number of valence electrons based on its position in the periodic table.
Once we know the total number of valence electrons, we distribute them around the central atom (M) and the surrounding atoms (G and S) to satisfy the octet rule. The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight valence electrons.
MGS Lewis Structure Octet Rule
The octet rule is a fundamental concept in chemistry that helps us understand the stability of atoms and molecules. According to the octet rule, atoms are most stable when they have a full outer shell of eight electrons. This can be achieved by gaining, losing, or sharing electrons through chemical bonding.
In the case of MGS, the central atom (M) will typically form covalent bonds with the surrounding atoms (G and S) to share electrons and achieve an octet. The number of covalent bonds formed by the central atom depends on the number of valence electrons it possesses.
MGS Lewis Structure Resonance
Resonance structures are alternative Lewis structures that represent the delocalization of electrons in a molecule. In some cases, a molecule can have multiple valid Lewis structures that differ only in the placement of electrons. These resonance structures contribute to the overall stability of the molecule.
When drawing the Lewis structure of MGS, it is important to consider the possibility of resonance. By examining the electron distribution and the connectivity of atoms, we can determine if resonance structures exist for MGS and how they contribute to its overall stability.
MGS Lewis Structure Lone Pairs
Lone pairs are pairs of valence electrons that are not involved in chemical bonding. In the Lewis structure of MGS, the central atom (M) may have lone pairs of electrons. These lone pairs affect the molecular structure and can influence the reactivity and properties of the molecule.
By considering the presence of lone pairs in the Lewis structure of MGS, we can better understand the electron distribution and predict the behavior of the molecule in chemical reactions.
MGS Lewis Structure Formal Charge
Formal charge is a concept used to determine the distribution of electrons in a molecule. It helps us assess the stability and reactivity of different resonance structures. In the Lewis structure of MGS, we can calculate the formal charge of each atom by comparing the number of valence electrons it possesses with the number of electrons it is associated with in the Lewis structure.
By analyzing the formal charges in the Lewis structure of MGS, we can identify the most stable resonance structures and gain insights into the electron distribution within the molecule.
MGS Hybridization
Hybridization is a concept that explains the mixing of atomic orbitals to form new hybrid orbitals. In the Lewis structure of MGS, the central atom (M) may undergo hybridization to accommodate the bonding and lone pairs of electrons. The type of hybridization influences the molecular geometry and the overall shape of the molecule.
By understanding the hybridization in the Lewis structure of MGS, we can determine the arrangement of atoms and predict the molecular shape and polarity.
MGS Lewis Structure Shape
The shape of a molecule is determined by the arrangement of atoms and lone pairs around the central atom. In the Lewis structure of MGS, the molecular shape is influenced by the number of bonding and lone pairs of electrons. The VSEPR (Valence Shell Electron Pair Repulsion) theory provides a framework for predicting the molecular geometry based on the repulsion between electron pairs.
By applying the VSEPR theory to the Lewis structure of MGS, we can determine its molecular shape and understand how it affects the physical and chemical properties of the molecule.
Deep Dive into MgS
MgS, also known as magnesium sulfide, is a chemical compound that consists of magnesium (Mg) and sulfur (S). In this deep dive, we will explore various aspects of MgS, including its chemical bonding, properties, and important reactions.
Is MgS Ionic or Covalent?
MgS is an ionic compound. Ionic bonding occurs between a metal and a non-metal, and in the case of MgS, magnesium is the metal and sulfur is the non-metal. The transfer of electrons from magnesium to sulfur results in the formation of an ionic bond.
What is the Difference Between Covalent and Ionic Bonding?
Covalent bonding, on the other hand, occurs between two non-metals. In covalent bonds, atoms share electrons to achieve a stable electron configuration. Ionic bonds involve the complete transfer of electrons, while covalent bonds involve the sharing of electrons.
Why MgS is Stored in Anhydrous Conditions?
MgS is stored in anhydrous conditions to prevent it from reacting with water. When exposed to moisture, MgS can react with water to produce hydrogen sulfide gas (H2S), which has an unpleasant odor. Therefore, it is important to keep MgS dry to maintain its stability.
What is the Chemical Name of MgS?
The chemical name of MgS is magnesium sulfide. It is composed of one magnesium atom and one sulfur atom, forming a 1:1 ratio.
Is MgS Soluble in Water?
MgS is sparingly soluble in water. It has a low solubility due to the strong ionic bonds between magnesium and sulfur. When MgS is added to water, it dissociates into magnesium ions (Mg2+) and sulfide ions (S2-). However, the solubility of MgS is limited, and only a small amount will dissolve in water.
Important Reactions of MgS
MgS can undergo various reactions due to its chemical properties. Here are some important reactions involving MgS:
-
Reaction with acids: MgS reacts with acids to produce hydrogen sulfide gas (H2S) and magnesium salts. For example, when MgS reacts with hydrochloric acid (HCl), it forms magnesium chloride (MgCl2) and hydrogen sulfide gas.
-
Reaction with oxygen: MgS can react with oxygen in the air to form magnesium oxide (MgO) and sulfur dioxide (SO2). This reaction occurs when MgS is heated or exposed to high temperatures.
-
Reaction with halogens: MgS can react with halogens, such as chlorine (Cl2), to form magnesium halides and sulfur. For example, when MgS reacts with chlorine gas, it forms magnesium chloride (MgCl2) and sulfur (S).
Practical Applications of MGS
MGS Uses
Molecular Geometry Software (MGS) is a powerful tool that has a wide range of practical applications in the field of chemistry. It helps chemists and researchers understand the three-dimensional arrangement of atoms within a molecule, which is crucial for predicting the molecule’s properties and behavior. Here are some common uses of MGS:
-
Lewis Dot Structure Visualization: MGS allows chemists to visualize the Lewis dot structure of a molecule, which shows the arrangement of valence electrons and helps determine the type of chemical bonding present.
-
Molecular Geometry Determination: By using MGS, chemists can determine the molecular geometry of a compound. This information is essential for understanding the spatial arrangement of atoms and predicting the molecule’s shape, polarity, and reactivity.
-
Prediction of Chemical Properties: MGS enables chemists to predict various chemical properties of a molecule, such as its bond angles, bond lengths, and molecular polarity. This information is crucial for understanding how the molecule will interact with other substances and participate in chemical reactions.
-
Analysis of Resonance Structures: MGS can be used to analyze resonance structures, which are different ways of representing a molecule’s electron distribution. This analysis helps chemists understand the stability and reactivity of the molecule.
-
Visualization of Molecular Models: MGS allows chemists to create visual representations of molecular models, which aids in understanding the overall structure and arrangement of atoms within a molecule. These models can be used for educational purposes or to communicate scientific findings.
MGS Stores
Apart from its uses, MGS also provides a convenient way to store and organize molecular information. Here are some features of MGS that facilitate efficient data storage:
-
Electron Distribution Database: MGS stores information about the electron distribution in various chemical compounds. This database allows chemists to access and compare electron configurations, atomic orbitals, and bonding patterns of different molecules.
-
Chemical Structure Repository: MGS serves as a repository for storing chemical structures, including structural formulas and chemical notations. Chemists can search and retrieve specific molecules based on their structural characteristics.
-
Hybridization Analysis: MGS provides tools for analyzing the hybridization of atoms within a molecule. This information is useful for understanding the bonding and geometry of the molecule.
-
Lone Pair Electron Tracking: MGS allows chemists to track the presence and location of lone pair electrons in a molecule. This feature is important for predicting the molecule’s reactivity and determining its overall shape.
Comparing MGS with Other Lewis Structures
MGCL2 Lewis Structure
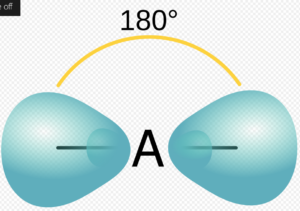
The MGCL2 Lewis structure represents the chemical bonding and electron distribution in magnesium chloride. In this structure, magnesium (Mg) forms a covalent bond with two chlorine (Cl) atoms. Magnesium has a valence electron configuration of [Ne]3s^2, while chlorine has a valence electron configuration of [Ne]3s^23p^5. By sharing its two valence electrons, magnesium achieves a stable octet configuration, while each chlorine atom also attains an octet by gaining one electron. The resulting structure is a linear molecule with a bond angle of 180 degrees.
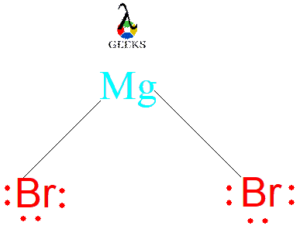
MG Br Lewis Structure
The MG Br Lewis structure illustrates the chemical bonding and electron distribution in magnesium bromide. In this structure, magnesium (Mg) forms a covalent bond with one bromine (Br) atom. Magnesium has a valence electron configuration of [Ne]3s^2, while bromine has a valence electron configuration of [Ar]3d^104s^24p^5. By sharing its two valence electrons, magnesium achieves a stable octet configuration, while bromine attains an octet by gaining one electron. The resulting structure is a linear molecule with a bond angle of 180 degrees.
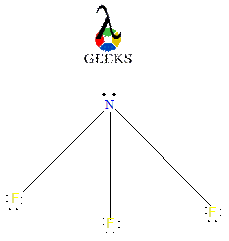
MG N Lewis Structure
The MG N Lewis structure represents the chemical bonding and electron distribution in magnesium nitride. In this structure, magnesium (Mg) forms a covalent bond with three nitrogen (N) atoms. Magnesium has a valence electron configuration of [Ne]3s^2, while nitrogen has a valence electron configuration of [He]2s^22p^3. By sharing its two valence electrons, magnesium achieves a stable octet configuration, while each nitrogen atom also attains an octet by gaining three electrons. The resulting structure is a trigonal planar molecule with a bond angle of 120 degrees.
Magnesium Sulfide Lewis Structure
The magnesium sulfide (MGS) Lewis structure represents the chemical bonding and electron distribution in magnesium sulfide. In this structure, magnesium (Mg) forms a covalent bond with one sulfur (S) atom. Magnesium has a valence electron configuration of [Ne]3s^2, while sulfur has a valence electron configuration of [Ne]3s^23p^4. By sharing its two valence electrons, magnesium achieves a stable octet configuration, while sulfur attains an octet by gaining two electrons. The resulting structure is a linear molecule with a bond angle of 180 degrees.
MG 2+ Lewis Structure
The MG 2+ Lewis structure represents the chemical bonding and electron distribution in a magnesium ion with a +2 charge. In this structure, magnesium loses its two valence electrons to achieve a stable octet configuration. The resulting structure is a cation with a +2 charge.
MGO Lewis Structure
The MGO Lewis structure illustrates the chemical bonding and electron distribution in magnesium oxide. In this structure, magnesium (Mg) forms a covalent bond with one oxygen (O) atom. Magnesium has a valence electron configuration of [Ne]3s^2, while oxygen has a valence electron configuration of [He]2s^22p^4. By sharing its two valence electrons, magnesium achieves a stable octet configuration, while oxygen attains an octet by gaining two electrons. The resulting structure is a linear molecule with a bond angle of 180 degrees.
MG CL Lewis Structure
The MG CL Lewis structure represents the chemical bonding and electron distribution in a magnesium ion with a -1 charge. In this structure, magnesium gains one electron to achieve a stable octet configuration. The resulting structure is an anion with a -1 charge.
By comparing the different Lewis structures mentioned above, we can observe variations in the number of covalent bonds formed, the number of lone pair electrons, and the resulting molecular geometry. These differences arise due to variations in the number of valence electrons and the electronegativity of the atoms involved.
It is important to note that Lewis structures provide a simplified representation of chemical bonding and molecular structure. They are based on the concept of the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight valence electrons. However, in some cases, molecules may exhibit resonance structures or violate the octet rule due to the presence of expanded octets.
To gain a more comprehensive understanding of molecular structure and chemical bonding, other theories such as the VSEPR theory and hybridization are often employed. These theories take into account the electron distribution and molecular shape, providing a more accurate representation of the actual molecular structure.
Understanding Lewis Structures in General
Lewis structures are a valuable tool in chemistry for understanding the arrangement of atoms and electrons in a molecule. They provide a visual representation of the valence electrons and help us predict the chemical bonding, molecular geometry, and overall structure of a compound. In this article, we will explore the fundamentals of Lewis structures and their significance in understanding chemical reactions and molecular properties.
How are Lewis Structures Written?
Lewis structures are written using a combination of chemical notation and symbols to represent the atoms and their valence electrons in a molecule. The process involves following a set of guidelines to determine the arrangement of electrons and the connectivity between atoms. To write a Lewis structure, we need to know the electron configuration of the atoms involved and understand the concept of valence electrons.
Valence electrons are the outermost electrons in an atom and play a crucial role in chemical bonding. They determine the reactivity and bonding behavior of an atom. In Lewis structures, valence electrons are represented as dots around the atomic symbol. Each dot represents one valence electron. For example, the Lewis dot structure of oxygen (O) would have two dots, indicating its six valence electrons.
How do Lewis Structures Work?
Lewis structures work based on the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight valence electrons. This rule is applicable to most atoms, except for hydrogen (H) and helium (He), which only require two valence electrons to achieve stability.
By following the octet rule, we can determine the number of bonds an atom can form and the overall electron distribution in a molecule. Covalent bonds are formed when atoms share electron pairs, and Lewis structures help us visualize these bonds by representing shared electrons as lines between atoms. For example, in a water molecule (H2O), the oxygen atom shares two electron pairs with two hydrogen atoms, resulting in two covalent bonds.
Why are Lewis Dot Structures Important?
Lewis dot structures are important because they provide insights into the molecular structure, chemical bonding, and electron distribution in a compound. They help us understand the arrangement of atoms and predict the properties of chemical compounds. By analyzing Lewis structures, we can determine the hybridization of atomic orbitals, identify resonance structures, and predict the polarity and molecular shape of a molecule.
Lewis structures are also essential in understanding chemical reactions. They allow us to visualize the breaking and formation of bonds during a reaction, helping us determine the reactants and products involved. Additionally, Lewis structures are used in the VSEPR (Valence Shell Electron Pair Repulsion) theory, which predicts the molecular shape based on the repulsion between electron pairs.
What are Lewis Structures?
Frequently Asked Questions
Is MGS Molecular?
MGS refers to Magnesium Sulfide, which is an ionic compound. Ionic compounds are formed through the transfer of electrons between atoms. In the case of MGS, magnesium (Mg) donates two valence electrons to sulfur (S), resulting in the formation of an ionic bond. Therefore, MGS is not molecular but rather ionic in nature.
Is MGS Ionic or Molecular?
As mentioned earlier, MGS is an ionic compound. Ionic compounds are composed of positively charged ions (cations) and negatively charged ions (anions) held together by electrostatic forces. In the case of MGS, magnesium (Mg) loses two valence electrons to form a positively charged ion (Mg^2+), while sulfur (S) gains two electrons to form a negatively charged ion (S^2-). The attraction between these oppositely charged ions results in the formation of an ionic bond, making MGS an ionic compound.
Is MGS a Molecular Compound?
No, MGS is not a molecular compound. Molecular compounds are formed through the sharing of electrons between atoms, resulting in the formation of covalent bonds. In contrast, MGS is composed of ions held together by ionic bonds. Ionic compounds, such as MGS, have a crystal lattice structure rather than discrete molecules. The Lewis dot structure and valence electrons play a crucial role in determining the type of chemical bonding present in a compound.
References
In the study of chemistry, understanding the structure and properties of molecules is crucial. The Lewis dot structure, also known as the electron dot structure, provides a visual representation of the valence electrons in an atom and is a fundamental concept in chemical bonding. By using dots to represent valence electrons, we can determine how atoms form covalent bonds and predict the molecular geometry of compounds.
The Lewis dot structure is based on the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with a full outer shell of eight electrons. This rule helps us understand how atoms bond together to form chemical compounds.
To determine the molecular geometry of a compound, we use the VSEPR (Valence Shell Electron Pair Repulsion) theory. This theory states that electron pairs, whether bonding or lone pairs, repel each other and arrange themselves in a way that minimizes repulsion. By considering the number of bonding and lone pairs around a central atom, we can predict the molecular structure and shape.
In addition to the Lewis dot structure and VSEPR theory, resonance structures play a significant role in understanding the bonding in certain molecules. Resonance occurs when multiple Lewis dot structures can be drawn for a molecule, indicating that the electrons are delocalized. This phenomenon is commonly observed in molecules with double bonds or lone pairs of electrons.
Chemists often use molecular models to visualize and study the three-dimensional arrangement of atoms in a molecule. These models help us understand the electron distribution, molecular shape, and overall chemical structure. By examining the hybridization of atomic orbitals and considering factors such as polarity and the presence of lone pair electrons, we can determine the structural formula and chemical notation of a compound.
Understanding the concepts of Lewis dot structures, valence electrons, chemical bonding, molecular geometry, and other related topics is essential for comprehending the atomic structure, chemical reactions, and properties of various chemical compounds. By applying these principles, chemists can predict the behavior and properties of substances, leading to advancements in various fields such as medicine, materials science, and environmental studies.
[]
Frequently Asked Questions
What is the Lewis structure in chemistry?
The Lewis structure in chemistry is a graphical representation of the arrangement of atoms in molecules and polyatomic ions. It represents the bonds between atoms, as well as the presence of lone pair electrons. The structure helps in understanding the type of bonding (covalent or ionic), molecular geometry, and the distribution of valence electrons, which play a crucial role in chemical reactions.
How are Lewis structures used in everyday life?
Lewis structures are used in everyday life to understand and predict the behavior of chemicals in various situations. For instance, they are used in the design of new drugs in pharmaceutical research, in the development of new materials in industrial chemistry, and in understanding environmental phenomena like ozone depletion. They help in visualizing the electron distribution and chemical bonding in molecules.
How are Lewis structures written?
Lewis structures are written by first determining the total number of valence electrons in the molecule or ion. Then, the atoms are arranged to show specific connections. Lines are drawn to represent bonds, each line representing a pair of bonding electrons. Remaining electrons are placed as lone pairs around the atoms. The structure should satisfy the octet rule, which states that atoms tend to combine in such a way that they each have eight electrons in their valence shells.
What is the Lewis structure for magnesium?
The Lewis structure for a magnesium atom represents its valence electrons. Magnesium has two valence electrons, which are typically represented by two dots around the symbol ‘Mg’. When magnesium forms compounds, it tends to lose these two electrons, becoming a Mg 2+ ion.
What is the Lewis dot structure for MGS?
The Lewis dot structure for MGS (magnesium sulfide) shows the transfer of two electrons from the magnesium atom to the sulfur atom, forming an ionic compound. The magnesium atom becomes a Mg 2+ ion and the sulfur atom becomes an S 2- ion. The structure illustrates the principle of chemical bonding in ionic compounds.
Is MGS molecular or ionic?
MGS, or magnesium sulfide, is an ionic compound. This is because it is formed by the transfer of electrons from the magnesium atom to the sulfur atom, resulting in positively charged Mg 2+ and negatively charged S 2- ions. These ions are held together by the strong electrostatic forces of attraction, known as ionic bonds.
What is the Lewis structure for MGCL2?
The Lewis structure for MGCL2 (magnesium chloride) shows that it is an ionic compound. The magnesium atom loses its two valence electrons to form a Mg 2+ ion. Each of the two chlorine atoms gains one electron to form Cl- ions. The structure illustrates the ionic bonding in this compound.
Why are Lewis dot structures important?
Lewis dot structures are important because they provide a visual representation of the arrangement of atoms in a molecule or ion, the type of bonds (covalent or ionic), and the distribution of valence electrons. They are fundamental to understanding the principles of chemical bonding, molecular geometry, and chemical reactions.
How does the Lewis structure work?
The Lewis structure works by representing atoms and their interactions. The central idea is that stability is achieved when an atom is surrounded by eight electrons (octet rule). The structure uses dots to represent valence electrons and lines to represent covalent bonds. It helps in predicting the molecular structure, polarity, and reactivity of the molecule.
What is the significance of resonance structures in Lewis structures?
Resonance structures in Lewis structures represent the delocalization of electrons within certain molecules or polyatomic ions where the octet rule is not obeyed. They are a set of two or more Lewis Structures that collectively describe the electron distribution in a molecule. Resonance structures help in understanding the stability, reactivity, and physical properties of the molecules.
Also Read: