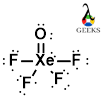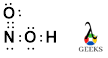The XeOF4 Lewis structure refers to the arrangement of atoms and electrons in the XeOF4 molecule. XeOF4 is a compound composed of xenon (Xe), oxygen (O), and fluorine (F) atoms. The Lewis structure helps us understand the bonding and electron distribution in the molecule. In the XeOF4 Lewis structure, xenon is the central atom bonded to four fluorine atoms and one oxygen atom. The oxygen atom forms a double bond with xenon, while the fluorine atoms form single bonds. This arrangement allows xenon to have an expanded octet, meaning it has more than eight valence electrons. Understanding the XeOF4 Lewis structure is crucial in predicting the molecule’s properties and reactivity.
Key Takeaways
| Atom | Number of Bonds |
|---|---|
| Xenon | 5 |
| Oxygen | 2 |
| Fluorine | 4 |
Understanding the Basics
To understand the concept of XeOF4 molecular geometry, it is important to first grasp the fundamental principles of chemical bonding and molecular structure. In this section, we will explore the key terms and concepts that form the foundation of this topic.
Definition of Key Terms
Before diving into the intricacies of XeOF4 molecular geometry, let’s familiarize ourselves with some important terms:
-
Xenon hexafluoride (XeOF4): A compound composed of one xenon atom and four fluorine atoms, arranged in a trigonal bipyramidal geometry.
-
XeOF4 polarity: Refers to the uneven distribution of electron density in the XeOF4 molecule, resulting in a partial positive charge on the xenon atom and partial negative charges on the fluorine atoms.
-
XeOF4 bond angles: The angles formed between the xenon atom and the surrounding fluorine atoms in the XeOF4 molecule. These bond angles determine the overall shape of the molecule.
-
XeOF4 hybridization: The process by which the atomic orbitals of the xenon atom in XeOF4 combine to form hybrid orbitals, allowing for the formation of bonds with the fluorine atoms.
-
Lewis dot structure: A diagram that represents the arrangement of valence electrons in a molecule using dots to represent electrons.
-
Valence shell electron pair repulsion theory (VSEPR theory): A theory that predicts the molecular geometry of a molecule based on the repulsion between electron pairs in the valence shell of the central atom.
-
Molecular orbital theory: A theory that describes the behavior of electrons in molecules by considering the overlap of atomic orbitals to form molecular orbitals.
-
Octet rule: A rule stating that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with eight valence electrons.
-
Xenon oxytetrafluoride (XeOF4): Another name for XeOF4, which highlights the presence of oxygen in the compound.
-
XeOF4 electron geometry: The arrangement of electron pairs around the xenon atom in the XeOF4 molecule, which is trigonal bipyramidal.
-
Chemical bonding: The process by which atoms combine to form molecules through the sharing or transfer of electrons.
-
XeOF4 molecular shape: The three-dimensional arrangement of atoms in the XeOF4 molecule, which is square pyramidal.
-
XeOF4 valence electrons: The electrons in the outermost energy level of the xenon atom that participate in chemical bonding.
-
XeOF4 resonance structures: Different Lewis dot structures that can be drawn for XeOF4, highlighting the delocalization of electrons.
These key terms provide a solid foundation for understanding the intricacies of XeOF4 molecular geometry. In the following sections, we will delve deeper into the concepts and theories that govern the structure and properties of this compound.
Lewis Structure of XeOF4

Detailed Explanation of XeOF4
XeOF4, also known as xenon oxytetrafluoride, is a chemical compound composed of xenon, oxygen, and fluorine atoms. Its Lewis structure helps us understand the arrangement of these atoms and the bonding within the molecule.
In the Lewis dot structure of XeOF4, we represent the valence electrons of each atom as dots around their respective symbols. Xenon (Xe) has 8 valence electrons, while oxygen (O) and fluorine (F) have 6 and 7 valence electrons, respectively. To determine the Lewis structure, we follow the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with 8 valence electrons.
Xenon, being a noble gas, already has a stable electron configuration. However, in XeOF4, it forms bonds with oxygen and fluorine to complete its octet. The Lewis structure of XeOF4 is as follows:
F
|
F - Xe - O
|
F
In this structure, the xenon atom is in the center, bonded to four fluorine atoms and one oxygen atom. The oxygen atom forms a double bond with xenon, while the fluorine atoms form single bonds. The Lewis structure helps us visualize the arrangement of atoms and the sharing of electrons in XeOF4.
Importance of XeOF4 in Chemistry
XeOF4 has significance in various areas of chemistry, including its molecular geometry, polarity, bond angles, hybridization, and resonance structures.
The XeOF4 molecular geometry is square pyramidal, with the xenon atom at the center and the fluorine atoms forming a square base around it. The lone pair of electrons on the oxygen atom gives the molecule its pyramidal shape. Understanding the molecular geometry is crucial in predicting the physical and chemical properties of XeOF4.
The polarity of XeOF4 arises due to the difference in electronegativity between xenon and oxygen. The oxygen atom is more electronegative, resulting in a partial negative charge, while the xenon atom carries a partial positive charge. This polarity gives XeOF4 its unique chemical behavior and reactivity.
The XeOF4 bond angles are approximately 90 degrees between the xenon atom and the fluorine atoms. These angles are determined by the repulsion between electron pairs, as predicted by the valence shell electron pair repulsion (VSEPR) theory. The VSEPR theory helps us understand the spatial arrangement of atoms and the bond angles in XeOF4.
In terms of hybridization, the xenon atom in XeOF4 undergoes sp3d2 hybridization. This hybridization allows the xenon atom to form bonds with the oxygen and fluorine atoms, resulting in the observed molecular structure.
XeOF4 also exhibits resonance structures, which are different representations of the Lewis structure that can be interconverted. These resonance structures help us understand the delocalization of electrons within the molecule and contribute to its overall stability.
Overall, the Lewis structure of XeOF4 provides valuable insights into its molecular shape, bonding, and properties. Understanding these aspects is essential for studying the chemical bonding and behavior of XeOF4 in various chemical reactions and applications.
Steps to Draw Lewis Structure of XeOF4

Calculation of Total Number of Valence Electrons
To begin drawing the Lewis structure of XeOF4, we first need to calculate the total number of valence electrons present in the molecule. Valence electrons are the electrons in the outermost shell of an atom and are involved in chemical bonding. In XeOF4, Xenon (Xe) is the central atom, and it belongs to Group 18 of the periodic table, also known as the noble gases. Noble gases have a full octet of electrons, meaning they have eight valence electrons. Oxygen (O) and Fluorine (F) are the other atoms in the molecule, and they also have their own valence electrons.
To calculate the total number of valence electrons in XeOF4, we add up the valence electrons of each atom:
- Xenon (Xe): 8 valence electrons
- Oxygen (O): 6 valence electrons
- Fluorine (F): 7 valence electrons (4 fluorine atoms in XeOF4)
Total valence electrons in XeOF4 = 8 + 6 + (7 x 4) = 8 + 6 + 28 = 42
Selection of the Centre Atom
The next step is to select the central atom in the Lewis structure of XeOF4. In this case, Xenon (Xe) is the central atom because it is the least electronegative atom in the molecule. The central atom is usually the atom that can make the most bonds with other atoms.
Connection of All Atoms to the Core Atom
After selecting the central atom, we connect all the other atoms to it. In the case of XeOF4, we connect the Oxygen (O) and Fluorine (F) atoms to the Xenon (Xe) atom. Each bond represents a pair of electrons shared between two atoms.
Understanding the Significance of Each Bond
The bonds in the Lewis structure of XeOF4 are important for understanding the molecular geometry and polarity of the molecule. The Xe-O bonds are polar due to the difference in electronegativity between Xenon and Oxygen. The Xe-F bonds are also polar for the same reason. The polarity of the bonds affects the overall polarity of the molecule.
Explanation of the Extended Octet of the Xenon Atom
Xenon (Xe) in XeOF4 exhibits an extended octet, meaning it can have more than eight valence electrons. This is possible because Xenon has empty d-orbitals in its valence shell, which can accommodate additional electrons. In XeOF4, Xenon forms bonds with four Fluorine atoms and one Oxygen atom, resulting in a total of 10 valence electrons around the Xenon atom.
Computation of Formal Charge
Formal charge is a concept used to determine the distribution of electrons in a molecule. It helps us understand the stability and arrangement of atoms in a Lewis structure. To calculate the formal charge of an atom, we compare the number of valence electrons it should have (based on its position in the periodic table) with the number of valence electrons it actually has in the Lewis structure.
Calculation of the Formal Charge on the XeOF4 Molecule
To calculate the formal charge on the XeOF4 molecule, we assign the valence electrons to the respective atoms and calculate the difference between the assigned electrons and the actual electrons present.
Formal charge on Xenon (Xe) = Valence electrons of Xe – Assigned electrons to Xe
Formal charge on Oxygen (O) = Valence electrons of O – Assigned electrons to O
Formal charge on Fluorine (F) = Valence electrons of F – Assigned electrons to F
Verification of the Derived Lewis Structure
Once we have assigned the formal charges, we need to verify if the derived Lewis structure of XeOF4 satisfies the octet rule and minimizes formal charges. The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable configuration with eight valence electrons. In the case of XeOF4, the Lewis structure should show Xenon with an extended octet and all other atoms with a complete octet. Additionally, the formal charges should be minimized as much as possible.
By following these steps, we can draw the Lewis structure of XeOF4 and gain insights into its molecular geometry, bond angles, and electron distribution. Understanding the Lewis structure of XeOF4 is crucial for studying its chemical bonding, molecular shape, and properties.
Advanced Concepts Related to XeOF4 Lewis Structure
XeOF4, also known as xenon oxytetrafluoride, is a compound that consists of a xenon atom bonded to four fluorine atoms and one oxygen atom. Understanding the advanced concepts related to the XeOF4 Lewis structure is crucial in comprehending its molecular geometry, hybridization, bond angles, resonance, and overall shape.
XeOF4 Hybridization
Hybridization refers to the mixing of atomic orbitals to form new hybrid orbitals that participate in bonding. In the case of XeOF4, the xenon atom undergoes sp3d2 hybridization. This means that the xenon atom’s 5p, 5s, and all three 5d orbitals participate in hybridization, resulting in six sp3d2 hybrid orbitals. These hybrid orbitals then form bonds with the surrounding atoms, giving rise to the molecule’s structure.
Geometry and Shape Calculation of XeOF4
To determine the geometry and shape of XeOF4, we can utilize the Valence Shell Electron Pair Repulsion (VSEPR) theory. According to VSEPR, electron pairs around the central atom repel each other and arrange themselves in a way that minimizes repulsion. In the case of XeOF4, the xenon atom is surrounded by four fluorine atoms and one oxygen atom.
The arrangement of these electron pairs leads to a square pyramidal geometry for XeOF4. The four fluorine atoms occupy the equatorial positions, while the oxygen atom occupies the axial position. This geometry gives XeOF4 a distorted tetrahedral shape.
XeOF4 Lewis Structure Resonance
Resonance structures are different representations of a molecule that can be drawn by moving electrons within the molecule. In the case of XeOF4, resonance structures can be drawn by moving the lone pairs of electrons on the oxygen atom to form double bonds with the xenon atom. However, it is important to note that resonance structures do not represent different forms of the molecule but rather contribute to its overall stability.
XeOF4 Lewis Structure Bond Angle
The bond angle in XeOF4 is an important characteristic that helps determine its molecular shape. In XeOF4, the bond angle between the xenon atom and the oxygen atom is approximately 90 degrees. This angle arises due to the repulsion between the lone pairs of electrons on the oxygen atom and the bonding pairs of electrons between the xenon and oxygen atoms.
XeOF4 Lewis Structure Molecular Geometry
The molecular geometry of XeOF4 is square pyramidal, as mentioned earlier. This geometry arises from the arrangement of the atoms and lone pairs around the central xenon atom. The four fluorine atoms occupy the equatorial positions, while the oxygen atom occupies the axial position. This arrangement gives XeOF4 its unique shape.
XeOF4 Lewis Dot Structure
The Lewis dot structure of XeOF4 represents the arrangement of valence electrons in the molecule. In XeOF4, the xenon atom contributes eight valence electrons, while each fluorine atom contributes seven valence electrons. The oxygen atom contributes six valence electrons. By distributing these electrons around the atoms and following the octet rule, we can construct the Lewis dot structure of XeOF4.
Comparisons and Contrasts
Comparison of XeOF4 and XeF4 Lewis Structures
When comparing the Lewis structures of XeOF4 and XeF4, we can observe some similarities and differences. Both molecules involve the element xenon (Xe) and fluorine (F), but the presence of oxygen (O) in XeOF4 sets it apart from XeF4.
In terms of molecular geometry, XeOF4 adopts a square pyramidal shape, while XeF4 has a square planar geometry. This difference in geometry is due to the presence of the lone pair on the oxygen atom in XeOF4, which causes distortion in the molecular structure.
The polarity of XeOF4 and XeF4 also differs. XeOF4 is a polar molecule due to the presence of the lone pair on the oxygen atom, which creates an uneven distribution of charge. On the other hand, XeF4 is a nonpolar molecule since the fluorine atoms are symmetrically arranged around the central xenon atom, resulting in a balanced charge distribution.
The bond angles in XeOF4 and XeF4 also vary. XeOF4 has a bond angle of approximately 90 degrees between the oxygen atom and the fluorine atoms, while XeF4 has bond angles of 90 degrees between the fluorine atoms.
In terms of hybridization, XeOF4 involves sp3d hybridization, while XeF4 involves sp3d2 hybridization. This difference in hybridization is due to the presence of the lone pair on the oxygen atom in XeOF4.
Comparison of XeOF4 and XeF2 Lewis Structures
Now let’s compare the Lewis structures of XeOF4 and XeF2. Both molecules contain xenon (Xe) and fluorine (F), but XeOF4 includes an oxygen (O) atom, while XeF2 does not.
In terms of molecular geometry, XeOF4 adopts a square pyramidal shape, while XeF2 has a linear geometry. This difference in geometry is due to the presence of the lone pair on the oxygen atom in XeOF4, which causes distortion in the molecular structure.
The polarity of XeOF4 and XeF2 also differs. XeOF4 is a polar molecule due to the presence of the lone pair on the oxygen atom, while XeF2 is a nonpolar molecule since there are no lone pairs and the fluorine atoms are symmetrically arranged around the central xenon atom.
The bond angles in XeOF4 and XeF2 also vary. XeOF4 has a bond angle of approximately 90 degrees between the oxygen atom and the fluorine atoms, while XeF2 has a bond angle of 180 degrees between the fluorine atoms.
In terms of hybridization, XeOF4 involves sp3d hybridization, while XeF2 involves sp3 hybridization. This difference in hybridization is due to the presence of the lone pair on the oxygen atom in XeOF4.
Comparison of XeOF4 and XeO4 2- Lewis Structures
Lastly, let’s compare the Lewis structures of XeOF4 and XeO4 2-. Both molecules contain xenon (Xe) and oxygen (O), but XeOF4 has four fluorine (F) atoms, while XeO4 2- has four oxygen atoms.
In terms of molecular geometry, XeOF4 adopts a square pyramidal shape, while XeO4 2- has a tetrahedral geometry. This difference in geometry is due to the presence of the lone pair on the oxygen atom in XeOF4, which causes distortion in the molecular structure.
The polarity of XeOF4 and XeO4 2- also differs. XeOF4 is a polar molecule due to the presence of the lone pair on the oxygen atom, while XeO4 2- is also a polar molecule due to the presence of two lone pairs on the central xenon atom.
The bond angles in XeOF4 and XeO4 2- also vary. XeOF4 has a bond angle of approximately 90 degrees between the oxygen atom and the fluorine atoms, while XeO4 2- has bond angles of approximately 109.5 degrees between the oxygen atoms.
In terms of hybridization, XeOF4 involves sp3d hybridization, while XeO4 2- involves sp3d2 hybridization. This difference in hybridization is due to the presence of the lone pair on the oxygen atom in XeOF4 and the additional lone pairs on the xenon atom in XeO4 2-.
Overall, the comparison of XeOF4 and XeF4 Lewis structures, XeOF4 and XeF2 Lewis structures, and XeOF4 and XeO4 2- Lewis structures reveals the impact of different atoms and lone pairs on the molecular geometry, polarity, bond angles, and hybridization. These comparisons highlight the importance of understanding the Lewis dot structures and the principles of chemical bonding, such as the octet rule, valence shell electron pair repulsion theory, and molecular orbital theory, in predicting and explaining the properties of molecules.
Frequently Asked Questions
What is the bond angle in the XeOF4 Lewis structure?
The bond angle in the XeOF4 Lewis structure is approximately 90 degrees and 180 degrees. This is due to the molecular geometry of XeOF4, which is square pyramidal.
Is the XeF4 Lewis structure polar or nonpolar?
The XeF4 Lewis structure is nonpolar. Despite the presence of polar bonds within the molecule, the overall structure is symmetrical, which results in the cancellation of the individual dipole moments.
What is the molecular geometry of XeOF4 according to its Lewis structure?
The molecular geometry of XeOF4 according to its Lewis structure is square pyramidal. This is determined by the Valence Shell Electron Pair Repulsion (VSEPR) theory.
How is the Lewis dot structure of XeOF4 represented?
The Lewis dot structure of XeOF4 is represented by having Xenon (Xe) in the center with four Fluorine (F) atoms and one Oxygen (O) atom surrounding it. There are also two lone pairs of electrons on the Xenon atom.
What is the hybridization of XeF4 in its Lewis structure?
The hybridization of XeF4 in its Lewis structure is sp3d2. This is due to the presence of six electron domains – four bonding pairs and two lone pairs.
What is the Lewis structure for XeF4?
The Lewis structure for XeF4 consists of Xenon (Xe) in the center surrounded by four Fluorine (F) atoms. Xenon also has two lone pairs of electrons.
What is the Lewis structure for CH3SOCH3?
The Lewis structure for CH3SOCH3 (Dimethyl Sulfoxide) consists of a central Sulfur (S) atom bonded to an Oxygen (O) atom and two CH3 groups. The Oxygen atom has two lone pairs of electrons.
Does XeF4 have resonance structures?
No, XeF4 does not have resonance structures. This is because all the Fluorine atoms are equivalent and there is no movement of electrons that would create different structures.
What is the shape of XeOF4 according to its Lewis structure?
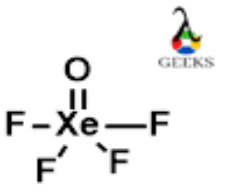
The shape of XeOF4 according to its Lewis structure is square pyramidal. This is determined by the Valence Shell Electron Pair Repulsion (VSEPR) theory.
Does XeF4 have an expanded octet?
Yes, XeF4 does have an expanded octet. The central Xenon (Xe) atom has 12 electrons around it – eight from the bonds with the Fluorine atoms and four from the lone pairs.
Also Read: