Difluoroethane (C2H2F2) has two carbon (C) atoms, each with 4 valence electrons, connected by a single bond. Each C atom is also bonded to one hydrogen (H) atom and one fluorine (F) atom. The Lewis structure shows four single bonds (two C-H and two C-F), with no lone pairs on the carbon atoms, using 18 valence electrons. C2H2F2 adopts a tetrahedral geometry around each carbon atom with bond angles close to 109.5°, characteristic of sp³ hybridization. The molecule is nonpolar overall, with polar C-F and C-H bonds due to electronegativity differences (C: 2.55, F: 3.98, H: 2.20). This structure influences its physical properties and reactivity, such as in refrigeration applications.

Difluoromethane is a haloalkane compound with two fluorine atoms. The strong carbon fluorine bonds in this molecule determines its chemical properties.
Facts about Difluoromethane
Difluromethane is an organic dihalo compound. It’s molecular formula is CH2F2. Two hydrogens atoms in methane is substituted by a fluorine atom. It have other names like difluromethylene, HFC- 32, methylene fluoride.
It is a colorless substance with gaseous nature. It has high thermal stability. But it’s boiling point and melting point is found to be very low that is -1360C and -520C respectively. It’s a substance with 52.024g/mol which has the ability to undergo endothermic process. It is used as a refrigerant and as a fire extinguisher.
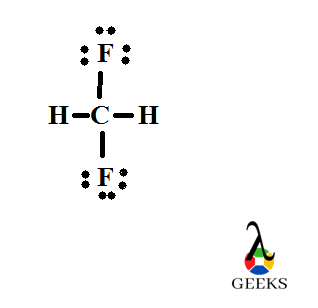
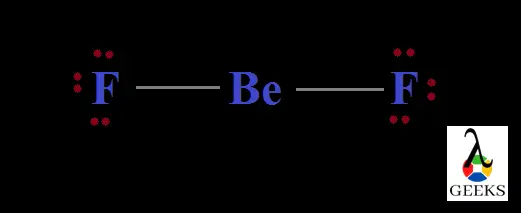
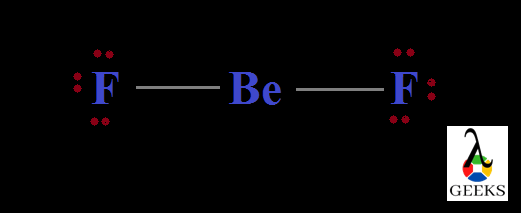
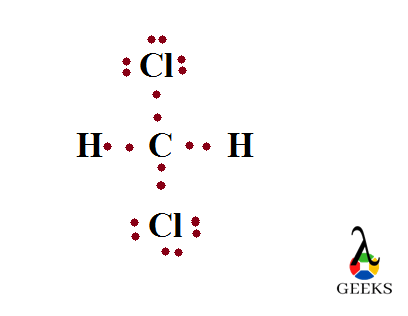
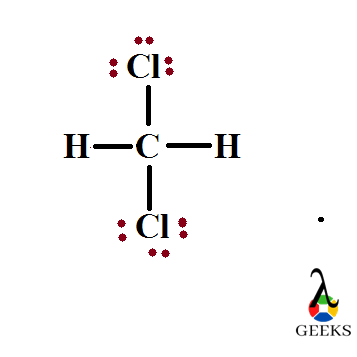
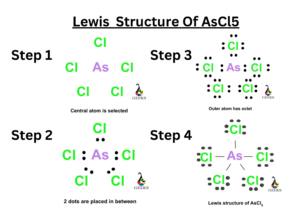
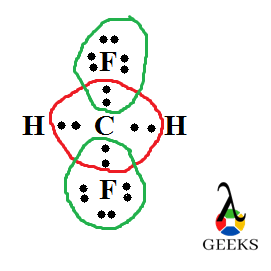
Lewis structure of Difluoromethane, CH2F2
Structures of molecules drawn through this concept is an easy way for understanding the bond formation between different atoms. Here the electrons and bonds are denoted by dots and lines respectively. So structures can be termed as Lewis dot structures.
Difluromethane is formed by the substitution of two hydrogen atoms by fluorine. The sum total of valence electrons in difluromethane is 20.
Carbon is the central atom and all the four other atoms are placed around it along with their valence electrons. Carbon forms four steady bonds with two fluorine and hydrogen.
Resonance in Difluoromethane, CH2F2
The motion of electrons in association with an atom results more than one structure to a molecule. Such structures are called resonance structure and process is resonance. Usually this can be seen in double bonded compounds. It is important to retain the electronic arrangements of atoms during resonance. There is no resonance structure found for difluromethane.
Difluoromethane, CH2F2 Octet Rule
We all know that difluromethane consists of one carbon attached with two hydrogen and fluorine. Carbon is the middle atom and has four outer electrons. Fluorine and hydrogen with seven and one outer electrons are present around carbon atom .
When the bond formation takes place carbon acquire four more electrons from Fluorine and hydrogen. So it’s valence shell now filled with eight electrons. Similarly fluorine has seven before bond formation and eight after bond making.
But hydrogen needs only two electrons for stable existence and it is obtained through bonds. So carbon and fluorine obeys octet rule. Even though hydrogen doesn’t obey octet rule it is stable.
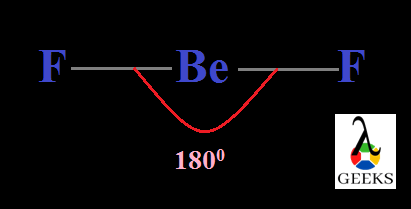
Difluoromethane, CH2F2 Shape and Bond angle
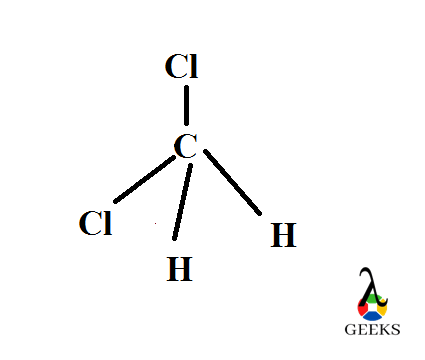
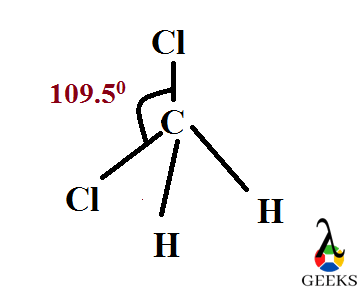
Molecules acquire different shape after their bond construction. They can have different shape dependent with the lone pairs present in the central atom. Here the central carbon has no lone pairs and is AX4 type. So it’s geometry is tetrahedral with an angle of 109.50 .
Here there are two different bonds. One is carbon- fluorine and latter is carbon- hydrogen. The polarity of former is much greater than the latter.

Difluoromethane, CH2F2 Lone pairs
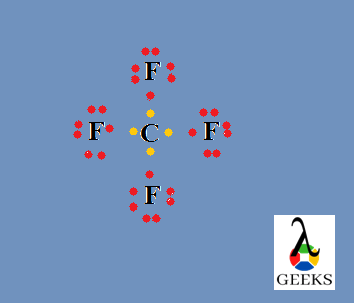
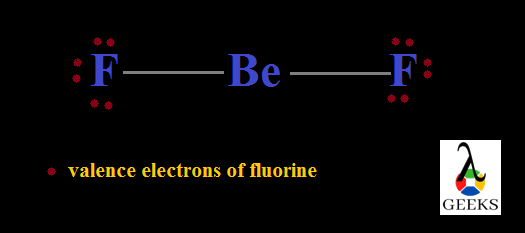
The number electrons which doesn’t take any role in bond formation is its lone pair of electrons. Here there is no lone pair for carbon and hydrogen. But there is lone pairs associated with two fluorine atoms. Out of seven outer electrons only one is used for bonding and the remaining six exist as three lone pairs. So total six lone pairs are present here.
Difluoromethane, CH2F2 Formal charge
The charge given to the atoms after bond construction is called formal charge. It can be either positive or negative and zero.
Formal charge = valence electrons – no. of dots – no. of bonds
Formal charge of carbon = 4-0-4 = 0
Formal charge of fluorine = 7-6-1 = 0
Formal charge of hydrogen = 1-0-1 =0
Formal charge of carbon, fluorine and hydrogen is zero.
Difluoromethane, CH2F2 Valence electrons
The total electrons seen in the outermost shell of any atom which has a major role in bond formation is called valence electrons. The total valence electrons in difluromethane is found by taking sum of valence electrons in each atom. So it is
Valence electrons in carbon – 4
Valence electrons in fluorine – 7
Valence electrons in hydrogen – 1
So total in Difluoromethane, CH2F2 is 4+7×2+1×2 = 20 electrons.
Difluoromethane, CH2F2 Hybridisation
Difluromethane is found to be a tetrahedral molecule in shape which follows sp3 hybridization. During sp3 hybridization the s and p orbitals of carbon atom overlapps to give a new set of sp3 orbitals with equal shape and energy. Then they combine with hydrogen and fluorine atoms orbitals shares its electrons with this sp3 orbitals to form bonds.

Difluoromethane, CH2F2 Solubility
Difluromethane is not much soluble in every solvents. One of the reason for this is its strong carbon- fluorine bond. There is vanderwaals dispersion force and dipole -dipole interactions exist in between difluromethane molecules.
When we try to dissolve this in water, there needs more energy to overcome these interactions. It has to overcome the energy due to hydrogen bonding between water molecules. So it is difficult to break this bonds and to create new one with the haloalkane and water.
Therefore it is sparingly miscible in water even though it is polar. But difluro methane is found to be soluble in ethanol.
Is Difluoromethane, CH2F2 Ionic or not ?
Difluromethane is a covalent compound formed by common sharing of electrons between carbon, hydrogen and fluorine. It follows sp3 hybridization. So it is not an ionic compound.
Is Difluoromethane, CH2F2 Polar or not ?
Difluromethane is a polar compound. The electronegativity of carbon, Fluorine and hydrogen is 2.55, 3.98, 2.20. Fluorine is the most electronegative atom so the electrons in carbon – fluorine bond is always towards fluorine atom.
So fluorine has partial negative charge and carbon has partial positive charge. So the carbon Fluorine bond is highly polarised. The electronegativity difference of carbon and hydrogen is 0.35 which is very low.
So it’s polarisation is low compared to C-F bond. Due to the high electronegativity difference seen in carbon- Fluorine and carbon- hydrogen bond difluromethane is found to be a polar molecule.
Is Difluoromethane, CH2F2 Acidic or Basic ?
The bond between carbon – fluorine is very strong so the bond can’t be broke very easily. Therefore difluro methane doesn’t undergo any type of chemical reactions. So it’s acidity and basicity cannot be distinguished.
Conclusion
Difluoromethane, CH2F2 is stable covalently bonded compound which is polar. It is because of strong carbon – fluorine bond. It follows sp3 hybridisation with tetrahedral shape and angle is about 109.50. Its Lewis structure is drawn here in this article. It has 20 valence electrons with six lone pairs around two fluorine atoms with zero formal charge.
Also Read: