In this article “Ionization energy examples” with their detailed explanations are derived. The meaning of higher ionization energy means facing the difficulty to subtract any electron from the chemical bonds.
11 + ionization energy examples are listed below,
- Fluorescent lamp
- Electrical bulbs
- Solvation
- Calcium nitride
- Free radicals
- Condensation reactions
- Sodium chloride
- Hydrogen
- Nitrogen
- Oxygen
- Aluminium
Fluorescent lamp:
Fluorescent lamp is a very light weight vapor lamp which inside mercury is present. In the fluorescent lamp fluoresce is placed for this reason visible light is delivered. Fluorescent lamp works in electric current. When electric current is deliver to the fluorescent lamp that time gas is energized the vapor of mercury from this ultraviolet radiation is emitted and the radiation of ultraviolet objective the coating of the phosphor in the liner wall of the lamp.

Image Credit – Wikipedia
Fluorescent lamp main parts:–
Electrodes:-
Inside the fluorescent lights two set electrodes are situated. The electrodes in fluorescent lights are attached with the fixture through two small size metal prongs. These prongs are clearly visible to the outside of the fluorescent lights. In the CFLs electrodes are not visible from the outer side because its base is screw type.
Starter:-
Only in the older type fluorescent lights have the component name starter. These starters are small metal cylinder. The starters of the fluorescent lights cause the delay of the electricity to the gas tube.

Image Credit – Wikipedia Commons
Tube:-
In the tube of the fluorescent light gas is placed. The usual fluorescent lights are tube shaped into the straight cylinder. In the CFLs, compact fluorescent light have a tube which is bending and look like U letter. In neon lights tubes are looks like words or graphics.
Gas:-
In the fluorescent light tube some gases are inset such as argon, xenon, neon and also vapor of mercury is placed. Gas of the fluorescent light is help to discharging the light. When a particular amount of voltage is applied the atoms of the ionized gas is charged and excited. At this moment proton of the ionized gas atoms also excited.
Phosphor coating:-
With the help of a metal named phosphor the inside tube of fluorescent light is coated. The coatings of the phosphor affect the colour emitting of the fluorescent light.
Ballast:-
In the fluorescent light the ballast can be two types one is electronic and another one is magnetic. In the new type of fluorescent light only the electronic ballast is present it is not too loud or hot like magnetic ballast.
Advantages of fluorescent lamp:-
- Low heat radiation
- Lower power consumption
- Longer life
- Not required warning up period
- Good quality of light
- Higher efficiency
Disadvantages of fluorescent lamp:-
- Initial cost is high
- Fluctuation of voltage is affected
- Produce radio interface
- Sometime light output is fluctuating
Electrical bulbs:
Electrical bulb is actually a simplest version of an electrical lamp. The electrical bulb is actually a very small size and simple light source which helps to brighten the dark place. The other name of the electrical bulb is incandescent bulb.
Application of Electrical bulb:-
- In portable lighting the electrical bulb is widely used such as table lamps
- In vehicle headlights and lights the electrical bulb is used
- Commercial lighting
- Household lighting
- Advertising and decoration the electrical bulb is used.
Advantages of Electrical blub:-
- No harassment in installation
- Longer life period
- Economical
- Affordable
- Easily available in verities shapes and sizes
- Working period is also high
- High output
Disadvantages of Electrical blub:-
- Energy insufficient
- Produce warm light
- Need to handle very carefully because it is made of glass thus can brake easily
- Breakable parts are very sharp can cut in the skin
- Inside the electrical bulb mercury, argon is present for this reason the electrical bulb should be handle carefully.
Solvation:
Materials which are made of plastic they are attacked by chemical reaction and salvation. The process method of salvation only happened with polar solvents. The concept of salvation is distinct from solubility and dissolution.

Image Credit – Wikipedia
Solvation can be explain as, the method in which chemical association is present between the solvent and molecules of solute.
The factors which are affecting solubility,
- Pressure
- Temperature
- Surface area
- ph
- Nature of Solvent/Solute
Read more about Pressure vessel : It’s important facts and 10+ applications
Calcium nitride:
The formula of the calcium nitride is Ca3N2. The molar mass of the calcium nitride is 148.25 gram per mol. The calcium nitride can present in a lots of state.
Free radicals:
A definition of free radical is any molecular house is capable to contain electron in unpaired state independently. Radicals can be present in two state one is unstable and another is highly reactive.
The radical can give an electron to other molecules or can take electron from other molecules. When the radical can give an electron to other molecules that time it behave like oxidants and when the radical can take an electron from other molecules that time it behave like reluctant.
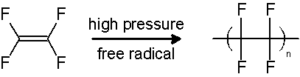
Image Credit – Wikimedia Commons
Some sources name of the free radicals is listed below,
- Exercise
- Smoking
- Mitochondria
- Inflammation
- Phagocytosis
- Ozone
- Radiation
- Pesticides
- Pollutions of the environments
- Industrial solvents
- Xanthine oxidase
Condensation reactions:
The condensation reactions are a part of chemical reactions. In condensation reactions smaller size molecules join together and make a larger size molecule. In the condensation reactions monomers means the smaller size molecules made a bond and name colavent bond and this bond is allow the joining the molecules and to make larger molecules.
The examples of condensation reactions are Glucose, Galactose.
The formula of condensation reactions is,
AH + BOH -> AB +H2O
Where,
A = The molecules in condensation reactions is condensed
B = The molecules in condensation reactions is condensed
AB = Compound product in condensation reactions
Sodium chloride:
The regular salt chemical name is sodium chloride. Sodium chloride is an electrolyte and helps to regulate the total quantity of water in our human body. But this chemical element also causes lot of problem in human body. They are listed below,
- Diseases of liver
- Diseases of kidney
- Congestive heart failure
- High blood pressure
- Fluid retention
Sodium Chloride preparation process:
When chloride and sodium mixed together then it is response to generate sodium chloride.
The formula in below,
2 Na (s) + Cl2 -> 2NaCl (s)
Hydrogen:
The hydrogen is a family member of chemical elements. It is a tasteless, colourless, odourless, flammable gaseous matter. The atom of the hydrogen contain a nucleus which surrounding have proton bearing which have the charge of positive electrical charge and an electron bearing is present which have the charge of negative electrical charge. In the whole universe hydrogen is one of the most abundant matters.
Three isotopes are present in the hydrogen. The isotope mass 1 is called protium and it symbol is H, and written as H1, The isotope mass 2 is called deuterium and a nucleus is present which contain one proton one neutron and it symbol is d, and written as H2, The isotope mass 3 is called tritium and it symbol is t, and written as H3 and its nucleus has one proton two neutrons.

Image Credit – Wikimedia Commons
Nitrogen:
In the periodic table of group 15 nitrogen is belonging. Nitrogen is lightest and non-metal of periodic table. In earth nitrogen is very important element. In the components of the nitrogen all proteins are placed. Nitrogen found in the system of living.
The atomic number of nitrogen is 7, atomic symbol is N. Most commons isotopes of nitrogen are Nitrogen – 14.
Applications of nitrogen:-
- Food packaging
- Manufacturing
- Aircraft fuel system
- Industry of light bulbs
- Fire suppression system
- Industry of chemical
- Tire filling system
- Manufacturing of electronics
- Manufacturing of stainless steel
- Fertilizer

Image Credit – Wikimedia Commons
Oxygen:
Pure form of oxygen is not flammable. It is a tasteless, colourless, odourless gaseous matter. In our surrounding some objects are present which are not burning in the air but can burn with the help of oxygen. Oxygen is very essential for our human life. The atomic number of oxygen is 8, atomic symbol is O.
It is reactive element and when it is added with another element oxide form. Oxygen could not make some oxide with some elements such as neon, argon, helium, and krypton.
Applications of oxygen:-
- Mining
- Rocket propulsion
- Production in glass industry
- Production in stone industry
- Medical field
- Biological field
- For melting and cutting of the metals
Aluminium:
In the table of the periodic aluminium is 13th element means the atomic number of the aluminium is 13. Aluminium is higher reactive and it is always ready to combine with other elements, for this reason in environment aluminium not present itself it presence can be observe with other elements.
Aluminium has high electrical conductivity for this reason it is used in the cables of the electric. Aluminium is silver metal which have extremely high corrosion resistant compare to others metals.
Application of aluminium:-
- Personal vehicle
- Construction of ships
- As a components in aircraft
- Window frames
- Power lines
- Household
- Industrial applications
- Construction of trains
- High rising building

Image Credit – Wikipedia
Frequent Asked Questions:-
Question: Ionization energy examples based on which equation?
Solution: The basic ionization energy equation is,
X(g) -> X (g) + e
More ionization energy equations,
1st equation of the ionization energy is,
X(g) -> X + (g) + e^-
2nd equation of the ionization energy is,
X(g) -> X2 + (g) + e^-
3rd equation of the ionization energy is,
X2(g) -> X3 + (g) + e^-