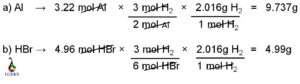
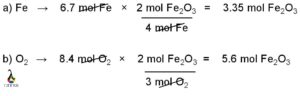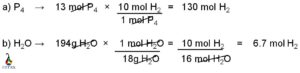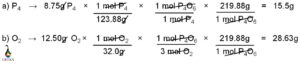In this article, “HCN hybridization” , hybridization, Lewis structure, bond connectivity of hydrogen cyanide with detailed explanation are discussed briefly.
Hydrogen cyanide known as prussic acid and it is a volatile, colorless and extremely toxic flammable liquid having a linear structure with a bond angle 1800. In this chemical species, carbon is sp hybridized having bond connectivity with hydrogen and nitrogen by two sigma and two pi bonds.
Few questions about the structure and hybridization of hydrogen cyanide are answered precisely below.
HCN Hybridization Structure
Hybridization of hydrogen cyanide can be easily explained by valance bond theory (VBT). Chemical bonding between the atoms can be determined by VBT. Like molecular orbital theory or MO theory, VBT also involved quantum mechanics.
According to this theory, bonding in any chemical species is the outcome of overlapping between the hybrid orbital of the consisting atoms. Two atoms share their half filled orbitals for overlapping to generate the hybrid orbitals. The two bond forming atoms have one unpaired electron and after overlapping between the orbitals two unpaired electron from each of the atom get paired up.
In HCN, carbon is connected with nitrogen by triple bond and hydrogen with a single or sigma bond. From this bond connectivity it is clear that carbon is sp hybridized and as a result hydrogen cyanide has a linear structure with a bond angle 1800. 2s and one 2p orbital of carbon atom participates in this hybridization. One sp hybrid orbital from carbon atom combines with the 1s orbital of hydrogen atom and another sp hybrid orbital overlaps with one of the p orbitals among three p orbitals of nitrogen atom which are left as unhybridized or pure p-orbital.

Image Credit: Wikimedia Commons
Hybridization of HCN can be calculated using the following formula.
- Hybridization= = GA + [VE – V – C]/2
- Hybridization of HCN = 2+ (4-4-0)/2 = 2
- GA= Groups of atoms attached to the central atom
- VE= Valance electrons of central atom
- V= Valency of central atom
- C= Positive or negative charge of the molecule.
To know more please check: 7 Tetrahedral Molecule Examples : Explanation And Detailed Facts
HCN Lewis Structure
Lewis structure or lewis dot structure helps to figure out the valance electrons or hybridization of any compound.
To determine the lewis structure of HCN, valance electrons of carbon, nitrogen and hydrogen should be counted from their electron configuration. Valance electron of hydrogen, carbon and nitrogen are 1, 4 and 5 respectively.
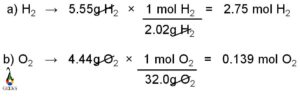
Central atom, carbon uses its three valance electrons to form triple bond with nitrogen and last valance electron for the single bond formation with hydrogen atom. Similarly, nitrogen uses its three valance electrons among the five outer most shell electrons to form the triple bond with carbon and rest of the two valance electrons remain as nonbonded electron pair. Hydrogen participates in the single bond formation with carbon by its one and only valance electrons.

To know more please follow: Is O2 a triple bond: Why, How, Characteristics and Detailed Facts
HCN Sigma and Pi bonds
From the point of hybridization the bond connectivity of central atom with each of the atom is clear. Central atom is carbon and it is sp hybridized with a linear structure. <HCN bond angle is 1800. Any covalent bonds is basically consisted of two electrons from each of the bond forming atoms.
Carbon is attached with nitrogen atom by three covalent bonds. Among these three bonds, one is sigma bond and another two is pi bonds. Carbon is also bonded with one hydrogen atoms by one sigma bond.
Sigma bond is the outcome of head on overlap of two atomic orbitals and pi bond is formed due to the lateral overlap of two atomic orbitals. Thus, sigma bond is much stronger than pi bond. The two pi bonds between carbon and nitrogen are formed due to the lateral overlap of two p orbitals (may be px and py or px and pz or py and pz). Rest of the p orbital (pz or py or px) participates in sigma bond formation with carbon.

To know more please check: 8+ Intermolecular Forces Examples: Detailed Explanations
HCN Polar or Nonpolar
Polarity of any molecule depends on the electronegativity difference of the atoms and the orientation of the respective atoms in that particular molecule.
We can consider the polarity of and direction of dipole moment of each of the bond. For the sigma bond between carbon and hydrogen, carbon is more polar than hydrogen (electronegativity of carbon is 2.55 and electronegativity of hydrogen is 2.2 in pauling scale). Thus, the direction of dipole moment is from hydrogen to carbon. If we consider the triple bond between carbon and nitrogen, we can see that the direction of dipole moment from carbon to nitrogen as nitrogen is more polar than carbon (electronegativity of nitrogen is 3.04 in pauling scale).
From the above explanation it is clear that HCN is definitely a polar molecule and its dipole moment is 2.9D.

Image Credit: Wikimedia Commons
To know more please go through: CH2CL2 Lewis Structure Why, How, When And Detailed Facts
Frequently Asked Questions (FAQ)
Is HCN soluble in water?
Answer: Yes, HCN is soluble in water because HCN is a polar molecule and water is also a polar solvent. Thus, HCN is soluble in water due to polar-polar interaction.
Is HCN covalent compound or ionic compound?
Answer: HCN is a covalent compound. Hydrogen is connected with cyanide ion by a single covalent bond and in CN– carbon is attached with nitrogen by three covalent bonds (1 sigma and 2 pi bonds).
Also Read: