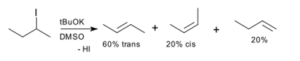
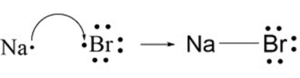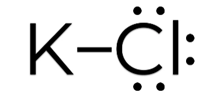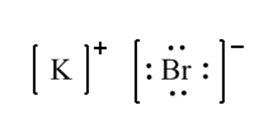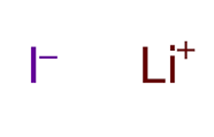Nitrogen gas (N2) is a diatomic molecule composed of two nitrogen atoms. When it comes to determining whether N2 is polar or nonpolar, we need to consider the electronegativity difference between the atoms and the molecular geometry. Polar molecules have an uneven distribution of charge due to the electronegativity difference between the atoms, while nonpolar molecules have an even distribution of charge. In the case of N2, the electronegativity of nitrogen is the same, resulting in a nonpolar molecule. This means that N2 has no positive or negative poles and does not exhibit dipole-dipole interactions. To further understand the polarity of N2, let’s delve into the concept of electronegativity and molecular geometry.
Key Takeaways
- N2 is a nonpolar molecule because it has a symmetrical linear shape and equal electronegativity between nitrogen atoms.
- Nonpolar molecules have no permanent dipole moment and do not have positive or negative poles.
- The electronegativity difference between atoms determines whether a molecule is polar or nonpolar.
- Understanding the polarity of molecules is important in predicting their physical and chemical properties.
N2: Polar or Nonpolar?

Explanation of polar and nonpolar molecules
When discussing the polarity of molecules, it is essential to understand the concept of electronegativity. Electronegativity refers to an atom‘s ability to attract electrons towards itself in a chemical bond. When two atoms with different electronegativities form a bond, the shared electrons are not equally distributed. This uneven distribution creates a separation of charge, resulting in a polar molecule.
On the other hand, in nonpolar molecules, the atoms involved in the chemical bond have similar or identical electronegativities. As a result, the shared electrons are evenly distributed, leading to a molecule with no separation of charge.
Comparison with HF molecule
To better understand the polarity of N2 (nitrogen gas), let’s compare it with the HF (hydrogen fluoride) molecule. HF is a polar molecule due to the significant difference in electronegativity between hydrogen and fluorine. Hydrogen has a lower electronegativity, causing the shared electrons to be pulled closer to the fluorine atom, resulting in a partial negative charge on fluorine and a partial positive charge on hydrogen.
In contrast, N2 is composed of two nitrogen atoms, which have the same electronegativity. Therefore, the electrons in the nitrogen-nitrogen bond are shared equally, resulting in a nonpolar molecule.
Explanation of N2 as a nonpolar molecule
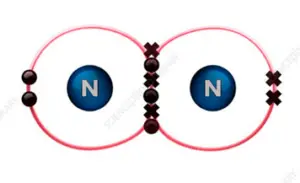
N2 is a diatomic molecule, meaning it consists of two nitrogen atoms bonded together. Each nitrogen atom has five valence electrons, and in the N2 molecule, these ten valence electrons are shared between the two atoms. The electron distribution in N2 is symmetrical, with each nitrogen atom contributing five electrons to the bond.
The equal sharing of electrons in the N2 molecule leads to a linear molecular shape. The two nitrogen atoms are directly bonded to each other, and the molecule has no lone pairs of electrons. This symmetrical arrangement of atoms and electrons results in a nonpolar molecule.
To determine the polarity of a molecule, we can also consider the dipole moment. The dipole moment is a measure of the separation of positive and negative charges in a molecule. In a nonpolar molecule like N2, the dipole moment is zero because there is no separation of charge.
Bond Type of N2
Nitrogen gas (N2) is a diatomic molecule composed of two nitrogen atoms. In order to understand the bond type of N2, we need to explore the nature of the bond between these atoms.
Explanation of Bond Types
When atoms come together to form molecules, they can do so through different types of bonds. The two main types of chemical bonds are covalent bonds and ionic bonds.
-
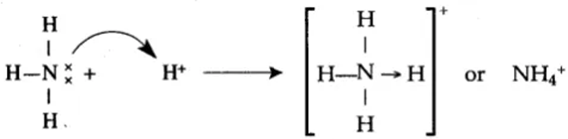
Covalent Bonds: Covalent bonds occur when atoms share electrons. This type of bond is typically formed between nonmetal atoms. In a covalent bond, the shared electrons are attracted to both nuclei, creating a strong bond.
-
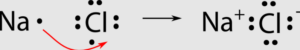
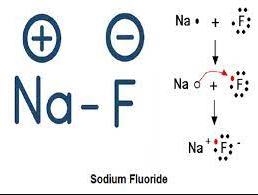
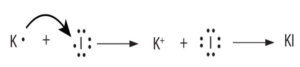
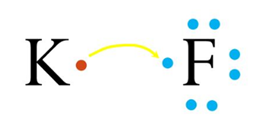
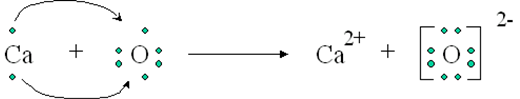
Ionic Bonds: Ionic bonds occur when there is a transfer of electrons from one atom to another. This type of bond is typically formed between a metal and a nonmetal. In an ionic bond, one atom becomes positively charged (cation) by losing electrons, while the other atom becomes negatively charged (anion) by gaining electrons. The attraction between these opposite charges creates the bond.
Determination of N2 Bond Type
To determine the bond type of N2, we need to consider the electronegativity of nitrogen atoms and the distribution of electrons in the molecule.
- Electronegativity: Electronegativity is a measure of an atom‘s ability to attract electrons towards itself in a chemical bond. The difference in electronegativity between two atoms can help determine the type of bond.
In the case of N2, both nitrogen atoms have the same electronegativity value since they are the same element. Nitrogen has an electronegativity value of 3.04 on the Pauling scale.
- Electron Distribution: In N2, each nitrogen atom has five valence electrons. To form a stable molecule, each nitrogen atom shares three electrons with the other nitrogen atom, resulting in a triple bond.
The triple bond in N2 consists of one sigma bond and two pi bonds. The sigma bond is formed by the overlap of two atomic orbitals head-on, while the pi bonds are formed by the sideways overlap of p orbitals.
Based on the electronegativity and electron distribution, we can conclude that the bond between the nitrogen atoms in N2 is a covalent bond. Since both nitrogen atoms have the same electronegativity, the electron pair is shared equally between them, resulting in a nonpolar covalent bond.
Summary
Molecular Geometry of N2
The molecular geometry of a molecule refers to the arrangement of its atoms in three-dimensional space. It provides crucial information about the shape and structure of the molecule, which, in turn, affects its physical and chemical properties. In the case of N2, or nitrogen gas, understanding its molecular geometry is essential in determining its polarity.
To understand the molecular geometry of N2, we need to delve into the VSEPR theory. VSEPR stands for Valence Shell Electron Pair Repulsion, and it is a model used to predict the shapes of molecules based on the repulsion between electron pairs in the valence shell of the central atom.
According to the VSEPR theory, electron pairs around the central atom will arrange themselves in a way that minimizes repulsion, resulting in specific molecular shapes. The electron pairs can be either bonding pairs (shared between atoms) or non-bonding pairs (also known as lone pairs).
Application of VSEPR Theory to N2
In the case of N2, the central atom is nitrogen (N), and it has a total of 10 valence electrons (5 from each nitrogen atom). Since N2 is a diatomic molecule, it consists of two nitrogen atoms bonded together by a triple bond.
To determine the molecular geometry of N2, we consider the electron pairs around each nitrogen atom. Each nitrogen atom has three bonding pairs, forming the triple bond, and no lone pairs. Therefore, the electron pair arrangement around each nitrogen atom is linear.
Explanation of Linear Molecular Geometry in N2
Based on the VSEPR theory, the linear electron pair arrangement around each nitrogen atom in N2 results in a linear molecular geometry for the entire molecule. This means that the two nitrogen atoms are aligned in a straight line, with a bond angle of 180 degrees.
The linear molecular geometry of N2 can be visualized as follows:
| Atom | Electron Pair Arrangement |
|---|---|
| N | Linear |
| N | Linear |
The linear molecular geometry of N2 has important implications for its polarity. Since the two nitrogen atoms are identical and the molecule is linear, the bond dipoles cancel each other out, resulting in a nonpolar molecule.
Electronegativity of N2
Definition of Electronegativity
Electronegativity is a fundamental concept in chemistry that refers to the ability of an atom to attract electrons towards itself in a chemical bond. It is a measure of the atom’s desire to gain electrons and form a stable electron configuration. The electronegativity of an atom is influenced by factors such as its atomic number, atomic radius, and electron configuration.
Electronegativity Value of Nitrogen
Nitrogen (N) is a nonmetallic element with an atomic number of 7. It is located in Group 15 of the periodic table and has five valence electrons. The electronegativity of nitrogen is 3.04 on the Pauling scale, which is a commonly used scale to measure electronegativity. This value indicates that nitrogen has a relatively high electronegativity compared to other elements.
Explanation of Nonpolar Nature of N2 based on Electronegativity
When considering the polarity of a molecule, it is essential to analyze the electronegativity difference between the atoms involved in the bond. In the case of nitrogen gas (N2), both nitrogen atoms have the same electronegativity value of 3.04. This means that there is no significant difference in electronegativity between the two nitrogen atoms.
Due to the equal sharing of electrons in the nitrogen-nitrogen bond, N2 is considered a nonpolar molecule. In a nonpolar covalent bond, the electrons are shared equally between the atoms, resulting in a symmetrical distribution of charge. As a result, there is no separation of positive and negative charges, and the molecule has no net dipole moment.
In the case of N2, the two nitrogen atoms share a triple bond, with each nitrogen atom contributing three electrons to form a total of six shared electrons. This sharing of electrons is equal and symmetrical, resulting in a nonpolar molecule. The Lewis structure of N2 further supports this, as it shows a linear arrangement of the atoms with no partial charges.
To summarize, the nonpolar nature of N2 can be explained by the equal electronegativity of the nitrogen atoms, leading to an equal sharing of electrons and a symmetrical distribution of charge. This absence of a net dipole moment makes N2 a nonpolar molecule.
| Property | N2 |
|---|---|
| Electronegativity | 3.04 |
| Molecular Shape | Linear |
| Dipole Moment | 0 |
| Lewis Structure | N≡N |
| VSEPR Theory | Linear |
Force of Attraction in N2
Nitrogen (N2) is a diatomic molecule composed of two nitrogen atoms bonded together. When discussing the force of attraction in N2, we need to consider the intermolecular forces that hold the molecule together. One of the primary forces at play in N2 is the London dispersion force.
The London dispersion force, also known as the dispersion force or the Van der Waals force, is a type of intermolecular force that exists between all molecules, including nonpolar molecules like N2. This force arises due to temporary fluctuations in the electron distribution within a molecule, creating temporary dipoles.
In the case of N2, each nitrogen atom has five valence electrons. These electrons are distributed in three bonding pairs and one lone pair. The electron distribution in N2 is symmetrical, resulting in a nonpolar molecule. However, despite being nonpolar, N2 still experiences London dispersion forces.
Explanation of London Dispersion Force in N2
The London dispersion force in N2 is a result of the temporary fluctuations in the electron distribution within the molecule. Even though N2 does not have a permanent dipole moment, the movement of electrons can create temporary dipoles. These temporary dipoles induce similar temporary dipoles in neighboring N2 molecules.
As a result, the temporary dipoles in N2 molecules attract each other, leading to a weak force of attraction. While the London dispersion force is generally weaker than other intermolecular forces, such as hydrogen bonding or dipole-dipole interactions, it still plays a significant role in determining the physical properties of N2.
The strength of the London dispersion force in N2 depends on factors such as the number of electrons and the shape of the molecule. In the case of N2, the presence of 14 valence electrons (7 electrons per nitrogen atom) contributes to a relatively strong London dispersion force.
Uses of N2
Nitrogen gas (N2) has a wide range of applications in various industries due to its unique properties. Let’s explore some of the common uses of N2 in different sectors.
Common applications of N2 in the chemical industry
In the chemical industry, nitrogen gas finds extensive use in various processes. Here are some of the common applications:
-
Blanketing and purging: N2 is often used to create an inert atmosphere in chemical reactors and storage tanks. By displacing oxygen and moisture, it helps prevent oxidation, degradation, and contamination of sensitive chemicals.
-
Solvent recovery: Nitrogen gas is employed in the recovery of solvents from chemical processes. It aids in the removal of volatile organic compounds (VOCs) and other impurities, allowing for the recycling and reuse of solvents.
-
Cryogenic applications: N2 is utilized in cryogenic processes, such as freezing and cooling. Its low temperature properties make it suitable for applications like cryogenic grinding, cryopreservation, and cryogenic distillation.
-
Chemical synthesis: Nitrogen gas is an essential component in the production of various chemicals, including ammonia, nitric acid, and urea. These chemicals serve as building blocks for fertilizers, explosives, and pharmaceuticals.
Use of N2 as a food preservative
Nitrogen gas plays a crucial role in the food industry, particularly in preserving the freshness and quality of perishable goods. Here’s how it is used:
-
Modified Atmosphere Packaging (MAP): N2 is commonly employed in MAP, a technique used to extend the shelf life of food products. By replacing the oxygen in the packaging with nitrogen, the growth of spoilage-causing microorganisms is inhibited, thereby preserving the food’s freshness.
-
Preventing oxidation: Nitrogen gas is used to prevent oxidative reactions in food products. It helps maintain the color, flavor, and nutritional value of packaged foods by reducing the exposure to oxygen, which can lead to spoilage.
-
Pressurizing and propelling: Nitrogen gas is utilized in the pressurization and propelling of aerosol cans used for food products like whipped cream. It helps create the necessary pressure for dispensing the product while ensuring its safety and quality.
Other industrial uses of N2
Apart from the chemical and food industries, nitrogen gas finds applications in various other sectors. Here are some notable examples:
-
Electronics manufacturing: N2 is used in electronics manufacturing processes, such as soldering, wave soldering, and reflow soldering. It helps create an oxygen-free environment, preventing oxidation and ensuring the quality of electronic components.
-
Oil and gas industry: Nitrogen gas is employed in oil and gas exploration and production. It is used for well stimulation, pressure testing, and as a lifting medium in enhanced oil recovery techniques.
-
Fire suppression systems: Nitrogen gas is utilized in fire suppression systems, particularly in areas where water-based systems may cause damage. It helps displace oxygen, effectively suppressing fires without leaving behind any residue.
-
Tire inflation: Nitrogen gas is increasingly used for tire inflation in various industries, including automotive, aviation, and racing. It offers benefits such as improved tire life, better fuel efficiency, and enhanced safety.
Frequently Asked Questions
Is N2 polar or nonpolar?
When it comes to the polarity of N2, it is considered a nonpolar molecule.
What forces does N2 have?
N2, also known as nitrogen gas, is held together by a strong covalent bond. The forces that hold the nitrogen atoms together in N2 are called covalent forces. Covalent bonds occur when atoms share electrons, resulting in a stable molecule.
Does N2 have polar bonds?
No, N2 does not have polar bonds. A polar bond occurs when there is an unequal sharing of electrons between two atoms. In N2, the nitrogen atoms share their electrons equally, resulting in a nonpolar bond.
What is the molecular geometry of N2?
The molecular geometry of N2 is linear. This means that the two nitrogen atoms are arranged in a straight line, with a bond angle of 180 degrees.
What is the order of electronegativity of N2?
Electronegativity is a measure of an atom‘s ability to attract electrons towards itself in a chemical bond. In the case of N2, both nitrogen atoms have the same electronegativity value, which is 3.04 on the Pauling scale. Therefore, the order of electronegativity for N2 is the same for both atoms.
Why is N2 nonpolar?
N2 is nonpolar due to its linear molecular geometry and the equal sharing of electrons between the nitrogen atoms. In a nonpolar molecule, the electronegativity difference between the atoms is either very small or nonexistent. Since the nitrogen atoms in N2 have the same electronegativity, there is no separation of charge, resulting in a nonpolar molecule.
Frequently Asked Questions
Is N2 a polar or nonpolar molecule?
N2 is a nonpolar molecule.
Why is N2 nonpolar?
N2 is nonpolar because it has a linear molecular shape and the two nitrogen atoms have equal electronegativity, resulting in a symmetrical distribution of electron density.
Is N2 polar or nonpolar or ionic?
N2 is neither polar nor ionic. It is a nonpolar molecule.
Is N2 ionic, polar covalent, or nonpolar covalent?
N2 is a nonpolar covalent molecule. It consists of a covalent bond between two nitrogen atoms.
What is the bond type of N2, polar or nonpolar?
The bond in N2 is a nonpolar covalent bond.
Which atom in N2 is closest to the negative side, polar or nonpolar?
In N2, neither nitrogen atom is closer to the negative side as the molecule is nonpolar.
Is the Lewis structure of N2 polar or nonpolar?
The Lewis structure of N2 is nonpolar.
Is N2 polar or nonpolar?
N2 is a nonpolar molecule.
What is the molecular shape of N2?
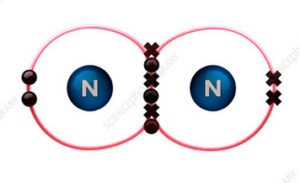
The molecular shape of N2 is linear.
What is the dipole moment of N2?
N2 has a zero dipole moment because it is a nonpolar molecule.
What is the electron distribution in N2?
In N2, the electron distribution is symmetrical due to the linear molecular shape and equal electronegativity of the nitrogen atoms.
What is the electronegativity of N2?
The electronegativity of N2 is equal for both nitrogen atoms since they are the same element.
 A+ + B– A is a cation with a positive charge and B is an anion with a negative charge.
A+ + B– A is a cation with a positive charge and B is an anion with a negative charge.