In this article “Aerobic septic system diagram” and aerobic septic system diagram related others facts will be discuss. Aerobic septic system diagram actually use components which are related to mechanical.
The aerobic septic system diagram is another form name is aerobic treatment system. In aerobic septic system a very small size sewage treatment system is work. It is almost same as septic tank system. In aerobic septic system mainly mechanical materials are used to treat discharge or sewage to an absorption area.
Read more about How Does A Heat Pump Work In Winter : Complete Insights, Critical FAQs
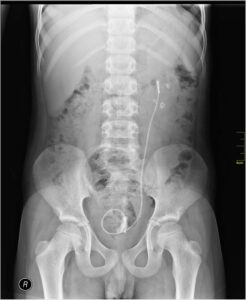
Septic pump system diagram:
Septic pump is systems contain a chamber which is placed in underground. It is made with fibreglass, concrete, plastic. In the chamber of septic pump system domestic sewage can flow for treatment.
Septic pump system diagram is given below,


Image Credit – Wikipedia
Aerobic vs. anaerobic septic system:
The major difference aerobic vs. anaerobic septic system is discuss below,
| Parameter | Aerobic septic system | Anaerobic septic system |
| By product | Aerobic septic system produce 1.Water 2. Excess amount of biomass 3. Carbon dioxide | Anaerobic septic system produce, 1. Excess amount of biomass 2. Carbon dioxide 3. Methane |
| Capital cost | Not too high | High |
| Maintenance cost | Expensive | Not too much expensive |
| Typical technologies | 1. Moving bed bioreactor 2. Activated sludge 3. Extended aeration 4. Tricking filters 5. DHS(Down flow hanging sponge) 6. Oxidation ditch 7. MBR(Membrane bioreactor ) | 1. Hybrid high rate reactor 2. Single stage UASB reactor 3. Two stage UASB reactor 4. Continuously stirred tank reactor 5. Continuously stirred tank up flow 6. Continuously stirred tank digester |
| Energy | High energy needed. | Low energy needed. |
| Volume of sludge | Large | Low |
| Hydraulic retention time | High | Low |
| Operation | Easy | Not easy |
| Structure | Simple | Complex |
| Process | In the aerobic septic system bacteria continuously supplied into the tank where oxygen is also present. The continuous flow of oxygen helps to keep the bacteria more effective and the treatment process can flow continuously. A movable pallet is placed in the tank thus wastewater can avoid splitting into different three layers. This process is easier to treatment wastewater. | In the system tank of the anaerobic two components are placed, one is treatment tank and another is seepage field. At first wastewater is delivering to the treatment tank. In the Treatment tank solid type waste is store in the bottom of the portion. In the top portion slag is placed and in the center of the tank waste water is placed. The wastewater is clean for this reason the water can flow by the pipes which are hidden placed into the leach area. Divider box is situated in the pipes. From the tank the wastewater flows more fluently. Before the step of filtration treated wastewater is back to the surrounding takes place at the point of leaching. |
| Effectiveness | More | Less |
Frequent asked question:-
Question: – Write the positive sides of Aerobic septic system diagram.
Solution: – The positive sides of Aerobic septic system diagram is listed below,
- Aerobic septic system reduce nitrogen
- Helps to decreasing drain field clogging
- Aerobic septic system perfect for water conservation
- Long life is high
- Continuously produce high quality wastewater
- Aerobic septic system produces clean effluent
- Aerobic septic system takes very less amount space for installation
- Aerobic septic system can establish in various types of soils
- Environmental friendly
- Maintenance is very low
- Simple design
Question: – Write the negative sides of Aerobic septic system diagram.
Solution: – The negative sides of Aerobic septic system diagram is listed below,
- The noise of the blower is too much excessive
- Installation cost is high
- More power draws
- Bad odour can comes if vented is not done properly
- Excessive amount of water is use in the Aerobic septic system
- Sometimes ammonia is emitted which could causes pollution in the nature.
- Aerobic septic system should be insulated neither in winter unfavorable condition will be appear
- High amount electricity is essential
- Frequent pumping needed
- Need more inspection
Question: – Describe the classifications of septic tank system.
Solution: – The size, shape, design can widely changes due to classified the septic tank system. The size factor of septic tank system included soil type, lot size, water bodies, weather condition and many more.
The classifications of septic tank system is listed below,
- Mound system
- Septic tank
- Chamber system
- Aerobic treatment unit
- Conventional system
- Drip distribution system
- Constructed wetland system
- Recirculating sand filter system
- Evapotranspiration system
- Community/Cluster system
Mound system:-
The most common classifications of septic tank system are Mound system. In the mound system tank is commonly used in high groundwater, soil depth and shallow bedrock. The mound system tank is made with sand and contained a drain field trench.
Wastewater is flow from septic tank to pump chamber. In the tank wastewater is pumped to the mound system that it could prescribed doses. After treatment of wastewater the water is discharge from the trench. After discharging the water is filter by sand and finally into the soil the water is disperses.
The mound septic tank system need periodic maintenance and a large amount space to install.
Septic tank:
The septic tank contained two chamber and these chambers are made of brick or concrete. PVC, pre or fibreglass septic tank, fabricated concrete rings is also available that will be less expensive.
The septic tank is small size scale decentralised treatment. The septic tank is actually a sedimentation tank and it is available in cylindrical or rectangular shape.
Advantages of septic tank:
- Electrical energy is not essential
- Little space required
- Low operating cost
- Long service life
- Simple construction
Disadvantages of septic tank:
- Only appropriate for low density housing
- Low reduction in solids, pathogens and organics
- Manual cleaning
Chamber system:
The chamber system type septic tank has series of chamber which are connected to each other. The chamber system septic tank area and its surrounding is filled with soil.
The chamber system septic tank has many types such as fabric wrapped pipe, open bottom chamber and many more.
Aerobic treatment unit:
Aerobic treatment unit is a natural process by which wastewater is treated. Oxygen rich bacterial is work for treatment in wastewater. For this process not too much space is needed and easy to construct.
Aerobic treatment unit process is done in four stages,
- Pre treatment
- Aeration chamber
- Disinfection
- Final treatment disposal
Conventional system:
In the conventional septic tank system which is used for very small size household or small business. The conventional septic tanks contain a septic tank and a bed subsurface or trench subsurface wastewater drain field system.
Drip distribution system:
Drip distribution septic tank system is used in many verities of drain fields. In the drip distribution septic tank system very large amount mound of soil is not needed.
This type of system maintenance cost is too high and electrical energy is needed.
Constructed wetland system:
The constructed wetland system treatment process is appearing in natural wetlands. This type of system can work in the pressure distribution or gravity flow.
Recirculating sand filter system:
Recirculating sand filter system is systems that can be construct both in below or above the ground. This type of system is very expensive to maintain and to install. Recirculating sand filter system is very high level treatment process system.
Evapotranspiration system:
Unique drain field type is evapotranspiration system. It is only useful for the specific environmental condition. The weather must be adequate sunlight and heat and must be arid.
Community/Cluster system:
Community/Cluster system the wastewater is collect from two or more than two buildings. This type of system mainly present in rural areas. Its contraction is not too much complicated.