In this article “Friction factor for turbulent flow” and friction factor for turbulent flow related several information will be discuss. The common method is to determine friction factor for turbulent flow is Moody diagram.
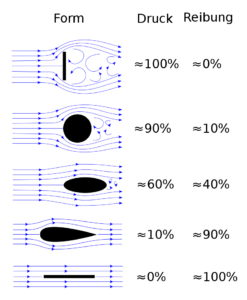
The friction factor is a physical parameter which is a dimensionless. The turbulent flow for a particular given type field is constant. The friction factor for turbulent flow only depends on the geometry of the channel and Reynolds number. The flow is called turbulent when Reynolds number is more than 3500.
What is the friction factor for turbulent flow?
Maximum system of the fluid in the nuclear facilities is work with the flow type of turbulent flow. The resistance of this flow obey the equation of Darcy – Weisbach.
The friction of the turbulent flow is measurement of the shear stress which is applied in the wall of a rod or pipe during the flow of turbulent. The flow of turbulent is obeying the equation of Darcy – Weisbach which is directly proportional to square of mean velocity of the flowing fluid in a certain area.
Turbulent flow:-
- When the value of Reynolds number is more than 3500 this type of flow called turbulent flow.
- Mathematical analysis of the turbulent flow is not too easy.
- Velocity of the turbulent flow is too high.
- Irregular movement is appearing in fluids which are flow in a motion in turbulent flow.
- Average motion is appearing in which side fluid is flowing.
- Turbulent flow in general very common type of flow.
- The velocity profile of the flow turbulent in a certain area is quickly drops when it comes to the wall of the pipe or rod.
- The velocity profile of the flow turbulent in a certain area is clearly flat when it comes to the center section of the rod or pipe.

Image Credit – Wikimedia Commons
Friction factor for turbulent flow formula:
The Colebrook–White equation is define as f for the Darcy friction factor, the function of for Reynolds number as Re, pipe relative roughness express as, ε / Dh for both smooth pipes and rough pipes.
The friction factor for turbulent flow formula is,
or,
Where,
Dh (m , ft) = Hydraulic diameter for filling the fluid in circular conduits
Dh = D= Inside diameter of the area from where flow of turbulent is flowing
Rh (m , ft) = Hydraulic radius for filling the fluid in circular conduits
Rh = D/4 = Inside diameter of the area from where flow of turbulent is flowing/4
The equation of Colebrook is solved by numerically for its implicit nature. Now a day Lambert W function is also use to obtain outspoken reformulation the equation of Colebrook.
or,
We will get,
px = ax + b
Expanded forms:-
Additional mathematical form of the equation of Colebrook is,
Where,
1.7384…. = 2 log (2 * 3.7) = 2 log (7.4)
18.574 = 2.51 * 3.7 * 2
And,
Or,
Where,
1.1364…. = 1.7384… = – 2 log (2) = 2 log
(7.4) – 2 log (2) = 2 log (3.7)
9.287 = 18.574/2 = 2.51 * 3.7
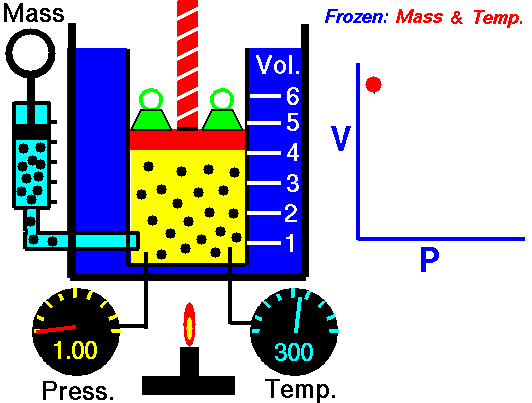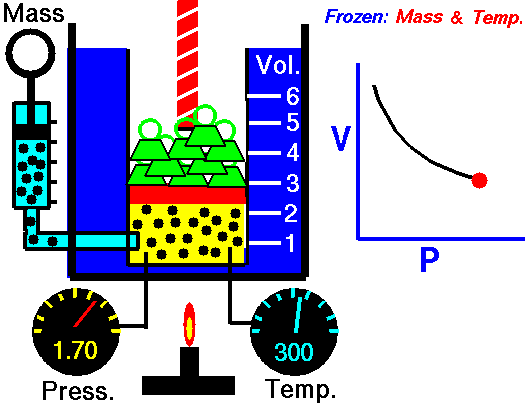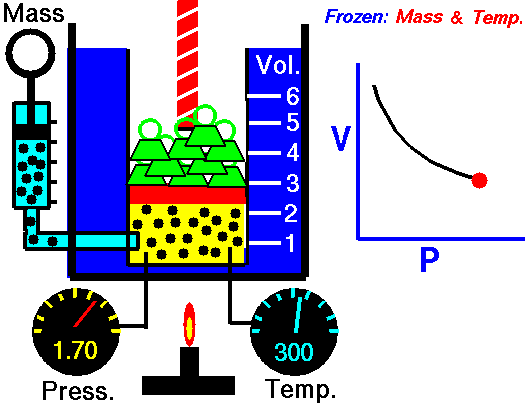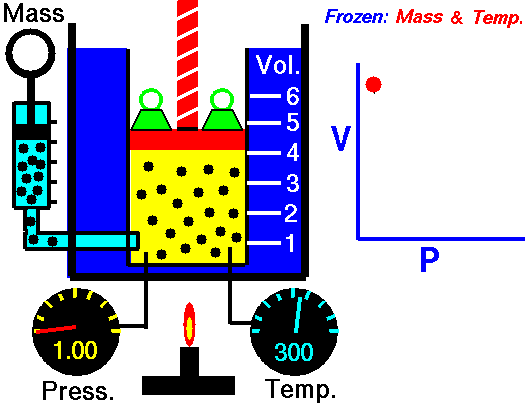
How to calculate friction factor for turbulent flow?
The process of calculating friction factor for turbulent flow is given below,
- At first we need to determine the value of Reynolds number for the turbulent flow using this formula,
- ρ x V x D x μ
- In the next step relative roughness should be calculated using k/D formula which value to under 0.01
- In the final step use the Moody formula for the roughness with the help of Reynolds number,
Friction factor for turbulent flow in pipe:
The range friction factor for turbulent flow in pipe is
For smooth pipe,
0.04 At Re 4000 to 1.01 at Re 3 x 106
For rough pipe,
0.045 At Re 4000 to 0.03 at Re 3 x 106
Friction factor for turbulent flow in smooth pipe:
Friction factor for turbulent flow in smooth pipe can be explained by the help of Blasius correlation. Blasius correlation is the simplest form of determine the Darcy friction factor.
Blasius correlation is only applicable for turbulent flow in smooth pipes it is not applicable for turbulent flow in uneven pipes. The value of 100000 of Reynolds number Blasius correlation is valid. In some cases of turbulent flow in uneven pipes is applied only because of its simplicity.
Mathematical equation of Blasius correlation turbulent flow in uneven pipes is given below,
After that the equation is corrected and express as,
With,
Where,
f is a function for the,
D = Pipe diameter express as meter, feet
R = Curve radius express as meter, feet
H = Helicoidal pitch express as meter, feet
Re = Reynolds number which is dimensionless
Reynolds number valid for,
0 < H/D < 25.4
Friction factor for turbulent flow in rough pipe:
The Darcy friction factor for turbulent flow in rough pipe means value of Reynolds number is more than 4000 is expressed by Colebrook – White equation.
or,
Where,
Dh (m , ft) = Hydraulic diameter for filling the fluid in circular conduits
Dh = D = Inside diameter of the area from where flow of turbulent is flowing
Rh (m , ft)= Hydraulic radius for filling the fluid in circular conduits
Rh = D/4 = Inside diameter of the area from where flow of turbulent is flowing/4
The equation of Colebrook is solved by numerically for its implicit nature. Now a day Lambert W function is also use to obtain outspoken reformulation the equation of Colebrook.
or,
We will get,
px = ax + b
Expanded forms:-
Additional mathematical form of the equation of Colebrook is,
Where,
1.7384…. = 2 log (2 * 3.7) = 2 log (7.4)
18.574 = 2.51 * 3.7 * 2
And,
Or,
Where,
1.1364…. = 1.7384… = – 2 log (2) = 2 log
(7.4) – 2 log (2) = 2 log (3.7)
9.287 = 18.574/2 = 2.51 * 3.7
Friction factors for turbulent flow in curved pipes:
In order to calculate the pressure drop with in a coil or pipe the friction factor should be calculated at first.
Friction factors for turbulent flow in curved pipes is discuss below,
D = Internal diameter of the coil or pipe
R = Darius of the coil or pipe helix
De = Dean Number
Rec = Transitional Reynolds number
fc = Friction factor of the coil or pipe which smooth
frough = Friction factor for a rough coil or pipe
fsmooth = Friction factor for a smooth coil or pipe
When a single phase flow is appear in a pipe or coil which shaped is curved a secondary flow pattern is introduce in the coil or pipe, in this time friction factor and fluid behaviour start to changes.
As the effect of stabilization of fluid flow the output is comes increases the Reynolds number at that point when flow is enter to the coil or pipe the transition flow form laminar flow to the flow of turbulent.
This condition mathematical form is given below,
To calculate the friction factor in a pipe or coil the Dean number is needed,
After this we easily can determine the friction factor for a smooth coil or pipe.
For,
De < 11.6
fc = 64/Re
For,
11.6 < De < 200
For, De > 2000
For calculation of totally turbulent flows determine the friction factor for a smooth coil or pipe using this equation,
The range is,
Moody diagram friction factor for turbulent flow:
In the flow of turbulent the relation between Reynolds number represent as Re, friction factor represent as fD, and relative roughness represent as ∈/D is complicated.
The expression for Moody diagram friction factor for turbulent flow is,

Image Credit – Wikimedia Commons
Frequent Asked Questions:-
Question:- Write about the Darcy friction factor chart.
Solution:- Darcy friction factor chart is combination of four physical parameters such as, pressure loss coefficient, Reynolds number, and relative roughness of the coil or pipe and diameter ratio of the coil or pipe.
Darcy friction factor chart is dimensionless physical factor with the help of Darcy – Weisbach equation can be written as,
Pressure drop can be calculate as,
Or,

Image Credit – Wikimedia Commons
The expression for Darcy friction factor for laminar flow is,
fD = 64/Re
In the flow of turbulent the relation between Reynolds number represent as Re, friction factor represent as fD, and relative roughness represent as ∈/D is complicated.