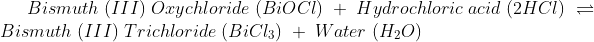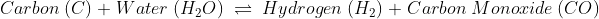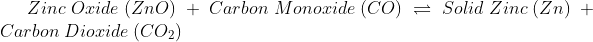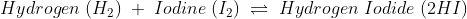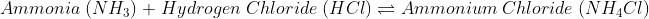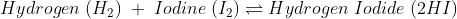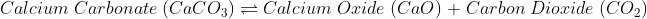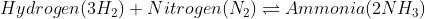The Chemical change is irreversible as new bonds are made.
When a chemical change occurs, the molecules present in one type of reactants break their bonds and make bonds with another kind of reactants, or they break bonds and create an entirely different molecule.
Due to this reason solely it is simple to understand why chemical change is irreversible. Though some chemical changes are reversible, that is because they have formed simple bonds that can be transformed back to the original. Whereas in irreversible chemical change, numerous complex changes occur that change the bond formation entirely, and it is impossible to reverse.
In this article, we shall have a close look at why chemical change is irreversible.
List of Contents
Creation of New Bonds
When two or more molecules react with each other, they create different types of bonds between them. These bonds, once created, are complicated to transform to the original. Though new molecules can be made by altering them, but they cannot be brought back to their initial state.
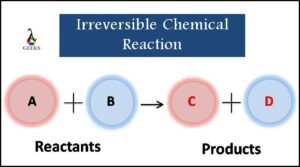
The molecules that react with each other are known as ‘Reactants,’ and the resultant molecules are known as ‘Products.’ Thus, in other words, reactants can be transformed into products, but products cannot be converted back to reactants.
Such equations are symbolized with an arrow that points in one direction.
Say, for example, reactant ‘A’ and reactant ‘B’ are made to react with one another, and they yield product ‘C’ and product ‘D,’ then the equation for this scenario is written as:
A+B→C+D
Thus, the chemical properties of the material are altered entirely when such reactions occur. The change in chemical properties also changes the physical properties of a substance at times. Hence, such reactions are difficult to reverse, and in some cases, even impossible to reverse.
Have a look at Irreversible Chemical Change Examples
Baking a Cake
For instance, let’s take the example of cooking. Say a person is baking a cake. For making a cake, they will need flour, egg, butter, salt, sugar, milk, baking soda, water, and edible decorating ingredients.
First of all, all these ingredients will be mixed together. We can’t even begin to imagine the chemical processes taking place inside a material when nine ingredients are mixed all together. After the mixture, the cake is baked. Baking, frying, sautéing, etc., change the properties of a material to a whole another level.
Now, once the cake is baked, there’s no way we can transform the cake back to the nine initial ingredients that we used.
Read more on Irreversible Process
Change in Energy
One of the most essential reasons why chemical change is irreversible is the energy difference. As we saw above that a chemical change occurs when bonds are broken or created.
Sometimes, there isn’t enough energy present in the system that can reverse a reaction, or there isn’t enough energy in the system that can break all the bonds in the products.
Here, energy does not necessarily mean heat. Nucleus, atoms, and molecules altogether have energy and energy levels of their own. Providing them external heat just won’t make the expected change sometimes.
Elimination of Reactant
When a chemical reaction is performed in an open system, more often than not, some amounts of reactants are lost to the environment. For example, elimination of oxygen (O2) or Carbon dioxide (CO2) as they are in gaseous form.
If the closed system is not appropriately maintained, the gaseous reactants or resultant products can also blow off in an open environment. A system in which there is no transfer of matter or energy, neither from inside nor from outside, is known as a closed system.
Though there are different closed systems in which the transfer of either of them is allowed, like, in thermodynamics, where transfer of energy is allowed, but the transfer of matter is not permitted in a closed system.
Consecutive Reactions
The formation of Carbon Tetrachloride from Methane involves many steps. Methane is chlorinated in the presence of light to obtain chloromethane, which is again chlorinated in the presence of light, giving dichloromethane. Further chlorination yields chloroform, chlorination of which will result in Carbon Tetrachloride.
The equation of the reaction is given as:
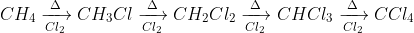
Chlorine is added to the products, and light works as a catalyst.
The initial reactants are the same, but the process is lengthy. Reversing this process is usually difficult or sometimes even impossible.
In this case, only chlorine is added at every step. There are also some reactions that involve more than two reactants at every stage, which makes the process even more complex.
Products are More Stable than Reactants
In some reactions, products are more stable than the reactants, and thus, transforming the products back to reactants is exceptionally burdensome. The products have double or triple bonds formed on occasions, which are not easy to break or even reverse.
Exothermic reactions are said to be irreversible. For example, combustion; when a log of wood is set to fire, it releases energy in the form of heat and light. This energy is emitted out of the system, and thus, it is an exothermic reaction. Hence, the products yielded will be more stable than the reactants, which is why we cannot bring back wood from ashes.
Also Read:
- Chemical equilibrium examples
- How chemical change occurs
- Chemical change examples
- Irreversible chemical change examples
- Chemical change types
- How can a chemical change be reversed
- Example of chemical change which is reversible
- Is chemical change reversible
- Is chemical equilibrium a dynamic equilibrium 2