We know about melting point and conductivity individually, but how melting point and conductivity relate to each other and their facts will be discussed in this post.
The melting point is the temperature at which a solid body begins to transfer its state into liquid. At the same time, conductivity is the temperature gradient to describe the heat transfer. Since both melting point and conductivity depend on the temperature, they share mutual relation.
Not every object has a melting point because some objects like wood do not melt. When it comes to conductivity, they are categorized based on the properties of the solid as thermal, electrical, ionic, etc. Here you will know the relation between melting point and thermal and electrical conductivity.
Melting point and thermal conductivity
Thermal conductivity is the ability of the compound to conduct heat. Generally, the flow of heat from higher to lower temperatures occurs in thermal conductivity. The thermal conductivity lies between the existence of the melting and boiling point of the given substance.
When the temperature rises through the heat transfer, the internal energy of the solid increases causing the diffusion, then the solid begins to melt. The melting point properties vary for metal and non-metal substances. The thermal conductivity in solid is due to the elastic vibration of lattice leads to the transfer of energy in the form of heat.
Melting point and thermal conductivity are individual properties of the substance, but in some applications like welding, the substance’s melting takes place through the thermal conductivity. Metals are more sensitive to temperature, so metal possesses a high melting point, and the thermal conductivity of the metal is more.

Melting point and thermal conductivity relationship
The melting point of the solids depends on their bond energy. If a solid has high bond energy, the melting point of the solid is also high. However, thermal conductivity depends only on the effective heat flow in the substance.
The melting point and thermal conductivity are closely related to certain solids, as both are temperature-dependent entities. The rise and fall of the temperature affect both melting point and conductivity.
In metals, the rise in temperature increases the melting point, consequently leading to a decrease in the thermal conductivity of the metal. This means that the melting point and thermal conductivity are inversely related in the case of metal. But for non-metal, it is converse. The melting point of the non-metal is lower, but its thermal conductivity is much higher.
This is evident that the melting point and thermal conductivity have a converse relationship.
Why is thermal conductivity inversely proportional to temperature?
The thermal conductivity of the solids largely depends on the motion of the particles’ free electrons and molecular vibration. The temperature change largely affects both of them; thus, thermal conductivity is inversely proportional to the temperature.
For better understanding, consider two cases of metals and non-metals as examples,
The thermal conductivity in metal is due to the motion of the free electron. As the temperature increases, the molecular vibration rises, leading to a decrease in the flow rate of a free electron by blocking its path, which consequently reduces the thermal conductivity.
The above theory is converse in the case of non-metal. Since there is no free electron in non-metals, the thermal conduction is due to molecular vibration. As the temperature rises, the molecules gain kinetic energy and cause molecule vibration; thus, thermal conductivity is high in non-metals.
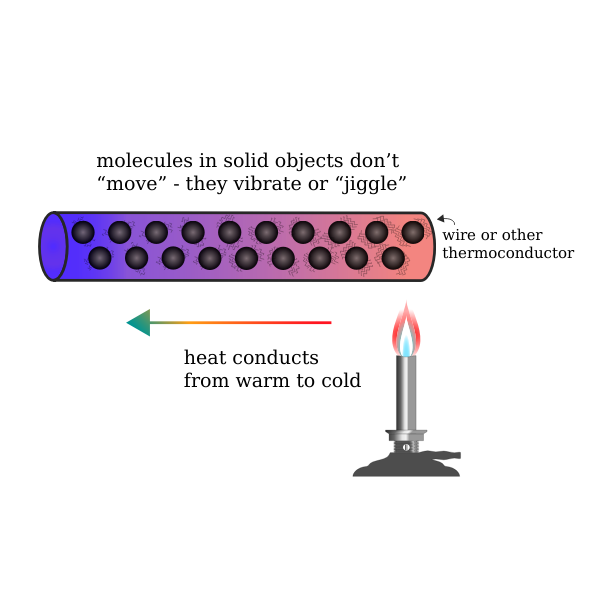
Image credits: Free SVG
Melting point and electrical conductivity
Electrical conductivity is the property of the material, which measures the ability to pass the current or to conduct electricity through the material. Since, to conduct electric current, a free electron is needed; hence only metals can be included in this case.
In the case of alkali metals, there is only one available free valance electron; hence energy needed to bind the atom in the crystal lattice is low, so the metallic bond is not so strong, which leads to a low melting point. But they are good electrical conductors.
The free valance electrons allow the electrical charges to flow freely and encourage the atoms to gain or lose one of the electrons from the element resulting from the weaker nuclear interaction. Thus electric conductivity is more.
Melting point and electrical conductivity relationship
Unlike thermal conductivity, the relationship between the melting point and electrical conductivity is linear. Since the increase in temperature leads to an increase in both electrical conductivity and melting point.
But, there are some consequences in which some metals have a high melting point, but their electrical conductivity is very low. Some metals are good electrical conductors but have low melting points. Thus it isn’t easy to establish a proper relationship between melting point and electrical conductivity.
What increases electrical conductivity?
Electrical conductivity relies on the temperature. Thus the variation of temperature can increase the temperature.
Electrical conductivity is highly influenced by the ion’s mobility and the valance electrons; thus, if there will be positive variation in the ionic mobility and valance electron, the electrical conductivity can be increased.
Since metals are good conductors, the electrical conductivity increases linearly if the number of free electrons is available per metal atom. If the delocalized electron increases, the electrical conductivity is also increased. For semiconductors, an increase in impurities can increase electrical conductivity.
Does melting point affect electrical conductivity?
For certain metals, the melting point largely affects the electrical conductivity. The electrical conductivity arises with the temperature. When the temperature reaches the melting point, it begins to fall, and thus electrical conductivity completely vanishes. This property is observed in the experiment of electric wire explosion carried by the phase transition process of metals.
The melting mechanism is enhanced by the deposition of energy on the crystalline lattice of the metal, leading to an increase in the number of high-energy electrons on the lattice, creating a defect in the lattice structure.
Since conductivity is the function of temperature, which also enhances the melting point, thus melting point affects the electrical conductivity.
What has a high melting point and does not conduct electricity?
Certain compounds have a high melting point but do not contribute to electrical conductivity. Those compounds are ionic. Ionic compounds in their solid-state do not exhibit electrical conductivity, but in their molten or aqueous state, they do.
In the solid-state, the molecules of the ionic compounds are held by a strong bond and fixed to their position. The possibility of forming free electrons is less, so they cannot move, but they have a high melting point, i.e., they melt so easily when some amount of temperature is supplied.
As the temperature rises, the ionic solid begins to melt, and ions become free to conduct electricity.
Does electrical conductivity increase with temperature?
The dependency of the electrical conductivity on temperature depends on the property of the material.
- For conductors, the temperature has an inverse relationship. Thus decreasing the temperature, the electrical conductivity can be increased.
- For insulators, the electrical conductivity can be increased by increasing the temperature.
- In the case of semiconductors, with the increase in temperature, the electrical conductivity of the semiconductor increases.
Since electrical conductivity is due to the free electron movement from one side to another, the electron is set to move freely if there is no resistance to the flow.
As the temperature rises, the vibration of the lattice causes the electron to achieve random motion in the lateral direction, so some resistance to the flow of electron exists; thus conductor exhibits low conductivity at high temperature.
High melting point and poor electrical conductivity
Certain metals such as hafnium, niobium, and tantalum exhibit high melting point, but they are poor electrical conductors. For example, tungsten has a high melting point but shows poor electrical conductivity in normal conditions.
However, tungsten is used as filament in bulbs because they exhibit electrical conductivity at a high temperature, allowing the flow of electrons.
The existence of impurities in the pure metal restricts the flow of electrons to conduct electricity; thus, even if they have a high melting point, they do not contribute to the electrical conductivity. Some of the compounds, such as stainless steel, have a relatively high melting point but do not conduct electricity because of their alloy-like structure.

Image credits: Pixabay
Why does conductivity increase with temperature in semiconductors?
The valance band is filled with valence electrons in semiconductors, and the conduction band is either empty or partially filled at zero degrees Kelvin. So the free electron to contribute electrical conductivity is unavailable in the conduction band to form electron-hole pair.
When a small amount of energy in the form of heat is applied, the electron can easily be available for conduction. As the temperature rises, the electron density in the conduction band increases. Thus conductivity is also increased in semiconductors.
The bandgap between the conduction and valance band is comparatively less; thus, the free electrons can cross the valance band due to thermal vibration as the temperature increases gaining kinetic energy. The electron becomes free within the lattice structure and naturally contributes to the electrical conduction.

Image credits: Wikimedia commons
Summary
Let us conclude this post by stating the melting point and conductivity are the properties of the material accompanied by the temperature. Thus they are indirectly correlated, and the conductivity varies differently for different compounds.
Also Read:
I am Keerthi K Murthy, I have completed post graduation in Physics, with the specialization in the field of solid state physics. I have always consider physics as a fundamental subject which is connected to our daily life. Being a science student I enjoy exploring new things in physics. As a writer my goal is to reach the readers with the simplified manner through my articles.

Hi Fellow Reader,
We're a small team at Techiescience, working hard among the big players. If you like what you see, please share our content on social media. Your support makes a big difference. Thank you!