If a system is not continuing its motion state or if its internal energy is not showing any change with respect to time then that system is said to be in an equilibrium. We can represent the walking of a person on a treadmill as an example here. Whenever a person is moving forward on a treadmill the treadmill will move in the opposite direction i.e,the backward direction to balance the person’s motion. It means that the person is in equilibrium with respect to the treadmill.
Now we will discuss 7+ interesting system in equilibrium examples.
- 1. A book on a table
- 2. A car that is moving with a constant velocity
- 3. A man who is standing on a horizontal plane
- 4. Dynamic equilibrium and its examples
- 5.Chemical equilibrium and its examples
- 6. Thermodynamic equilibrium and its examples
- 7. Static equilibrium and its examples
1. A book on a table
If we keep a book on a table then that book will be in equilibrium. Why? Because the sum of the net forces that are acting on that book remains zero and the sum of total torques that are acting on that book is also zero. As these two main conditions are satisfied here that is why that book is in equilibrium. In this case the linear acceleration and the angular acceleration both are also zero.
∑F=0,∑τ=0
2.A car that is moving with a constant velocity
A truck that is moving with a constant velocity is another important and common system in equilibrium examples. Now the question arises how a moving body can be in equilibrium? The answer is : this type of equilibrium is dynamic equilibrium. It means that in this case also the sum of total forces is zero and the sum of total torque is also zero. ∑F=0,∑τ=0.
In this case the linear acceleration and the angular acceleration are also zero. Hence the net effects of forces and torques are zero. That is why the truck which was moving with a constant velocity keeps moving with the same velocity and there is no change in its velocity. So there is no linear as well as angular acceleration.
3. A man who is standing on a horizontal plane
A man who is standing on a horizontal plane is an example of a system in equilibrium. We all know that when a man is standing upon a horizontal plane then his weight is acting in the downward direction. The weight of the man can be defined as the force acting downwards due to the effect of gravity. This weight or gravitational pull is balanced by the reaction force which is acting in the opposite direction of the gravitational pull.
In this way two forces,weight acting downwards and reaction force acting upwards are balanced by each other. Hence the main conditions of a system in equilibrium are satisfied. The conditions are ∑F=0,∑τ=0. This is the reason why a man on a horizontal plane remains in equilibrium.
4. Dynamic equilibrium and its examples
Let us take a body which is moving at a constant velocity. If the body keeps moving without any kind of external disturbance then that body will be said to be in equilibrium. Why? Because we know that if there is no effect of any external force on a body it will keep moving at the same velocity as earlier. This law is known as Newton’s second law of motion.
So dynamic equilibrium is related to Newton’s second law of motion. Now what are examples of dynamic equilibrium that are observed everyday in our daily life? The examples are a car moving at a constant velocity and a bucket of water that is being raised at a constant velocity by a pulley rope system.
In both of these examples that are mentioned above we have seen that the sum of the net forces on that car as well as the bucket of water is zero,hence both the car and the bucket are moving at a constant velocity. Hence dynamic equilibrium is maintained here.
5.Chemical equilibrium and its examples
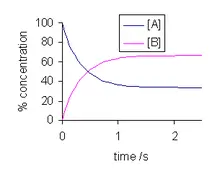
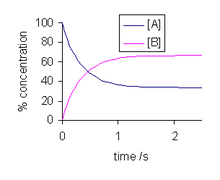
There are so many questions related to chemical equilibrium like how it can be achieved,when it can be achieved etc. First of all the reaction should be a reversible reaction. If in a reversible chemical reaction the rate of forward reaction becomes equal to the rate of reverse reaction then a system achieves chemical equilibrium.
Now we have to know what is forward reaction and what is reverse reaction. Basically when products are produced from the reactants in a chemical reaction then this reaction is known as forward reaction. When reactants are formed from products in a reversible chemical reaction then that reaction is known as reverse reaction. FORWARD REACTION: REACTANTS → PRODUCTS
REVERSE REACTION: PRODUCTS → REACTANTS
To describe chemical equilibrium we will take the help of an example of a bottle of cold drink. In this bottle CO₂ gas is dissolved in the liquid and also it is in gaseous form in the gap between the cap and the surface of the liquid inside the bottle. This gas continuously changes into liquid as well as the liquified gas also turns into gas in the bottle. That means the system is in chemical equilibrium.
CO₂↑ + H₂O⇋H₂CO₃
Another common example is haemoglobin that transports oxygen within our bodies.
6. Thermodynamic equilibrium and its examples
If I connect two containers so that heat can flow from the hot container to the cold one. If there is no flow of heat between two containers,it means that both of them are in thermodynamic equilibrium with each other.
Through a simple example we can describe thermodynamic equilibrium. Say there is a bottle in which a gas is kept and there is a movable piston in it. If the temperature and pressure that are maintained within the container are kept uniform then this container will be in thermodynamic equilibrium.
Another condition is there which must be followed. The external forces acting upon the movable piston should not be able to move it. In this case ΔT=0,ΔP=0. Here ΔT is the change in temperature and ΔP is the change in pressure.
7. Static equilibrium and its examples
A book that is kept on a table,a rigid body kept on a horizontal surface all these are the examples of static equilibrium. in these cases the sum of total force is zero and the sum of total torque is zero.
Some additional points related to the system in equilibrium
In the case of a body which is in equilibrium there will be no existence of linear acceleration and angular acceleration. If there is no effect of any external force present in that system then its state of equilibrium will be continued indefinitely. This will be perturbed only if there is an effect of any external force acting upon the system.
If we take an example of a rigid body then for that body if the sum of all the torques that are acting on it is zero,then only the body is said to be in equilibrium. ∑F=0,
∑τ=0.This happens because in this way the rotational motion remains unchanged. If there is any displacement taking place in a body then if there is any action of a force that can oppose this effect and again turn the body to equilibrium then this type of equilibrium is known as stable equilibrium.
Conclusion
In this article we have discussed different system in equilibrium examples. We have also discussed different types of equilibrium that are dynamic equilibrium,chemicalequilibrium,thermodynamic equilibrium in a brief manner equipped with some of their common examples.
If a person wants to gain some knowledge regarding a system in equilibrium he or she can go through this article to have a clear picture of that system in equilibrium.
Also Read: