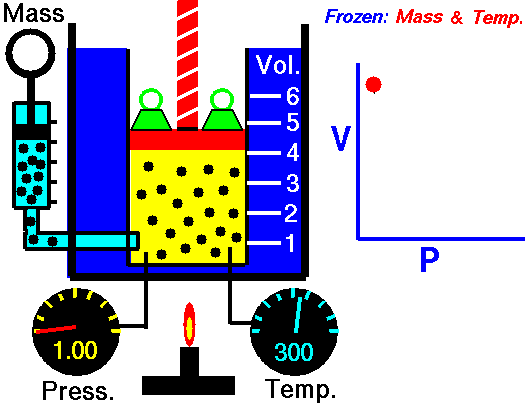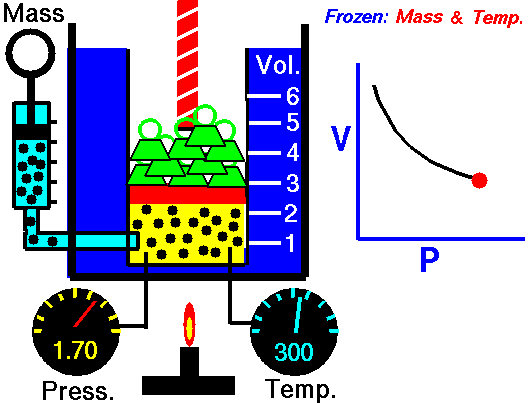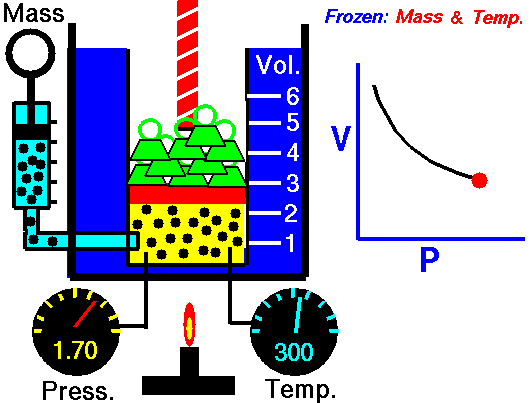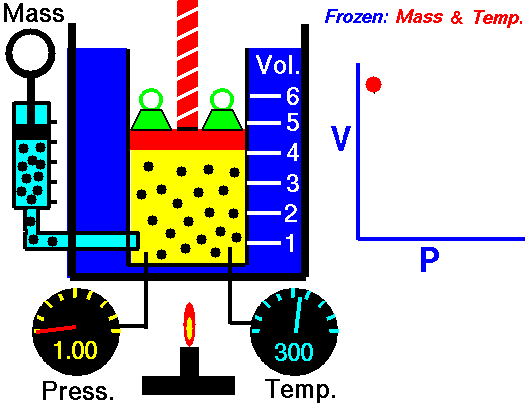
In this article “Thermal equilibrium example” will be discuss with detailed explanations. Thermal equilibrium example is very important concept to understand the changing of state for a substance.
11+ Thermal Equilibrium Example is encounter in below,
- Refrigerator
- Oven
- Melting of a ice cube
- Freezing of water
- Drying of wet hair
- Drying of wet clothes
- Cooling of a cup of tea
- Melting of ice-cream
- Freezing of ice- cream
- Cooling of a hot rod
- Making of tea
Refrigerator:-
Refrigerator is a device which is appropriate example of thermal expansion. When a food item is stored in the refrigerator that time the item will start to goes down its temperature and the temperature of the refrigerator and the temperature of food item will be same and the process of temperature changing for the food item will be stop.
Refrigerator is a home appliance and commercial appliance. The refrigerator carry a compartment with thermal insulator and also a heat pump through which heat easily can transfer from its midst to its external surrounding. For this process the inside temperature of the refrigerator will be lower than the temperature of the room.

Image Credit – Wikimedia Commons
Oven:-
Oven is a device which is also an appropriate example of thermal expansion. When a food item is placed in an oven and heat is applied on it that time the item will start to goes up its temperature. When the temperature of the oven and the temperature of food item will be same that time the process of temperature changing between the oven and the food item will be stop.
An oven is use for cooking purpose and also heats the food item to a wished temperature.

Image Credit – Wikimedia Commons
Types of oven:
Types of oven is listed below,
- Electric oven
- Gas oven
- Earth oven
- Masonry oven
- Toaster oven
- Ceramic oven
- Wall oven
- Steam oven
- Microwave oven
Melting of a ice cube:-
Another example of thermal expansion is melting of an ice cube. When an ice cube placed on a normal temperature its try to reaching at the room temperature and melting point will be increases at this particular time ice cube start to changing its state from solid to liquid.

Image Credit – Wikimedia Commons
Freezing of water:-
Another example of thermal expansion is freezing of water. When water is placed on a lower temperature its try to reaching at the lower temperature from the normal temperature at this particular time water starts to change its state from liquid to solid.
Drying of wet hair:-
Drying of wet hair is another regular example of thermal expansion. When we dry our wet hair in normal room temperature that time our wet hair reach at the room temperature and hair will be dry.
Drying of wet clothes:-
Drying of wet clothes is another regular example of thermal expansion. When we dry our wet clothes in normal room temperature that time our wet clothes reach at the room temperature and cloth will be dry.
Cooling of a cup of tea:-
Cooling of a cup of tea is another regular example of thermal expansion. When a cup of tea is placed on a normal room temperature that time a cup of tea try to reaching at the room temperature and boiling point will be decreases. A cup of tea starts to change its temperature and became cool.

Image Credit – Wikimedia Commons
Melting of ice-cream:-
Another example of thermal expansion is melting of ice cream. When ice cream is placed on a normal room temperature that time its try to reaching at the room temperature and melting point will be increases and freezing point will be decreases. The ice cream starts to change its state from solid to liquid.

Image Credit – Wikimedia Commons
Freezing of ice- cream:-
Another example of thermal expansion is freezing of ice cream. When ice cream is placed on a refrigerator that time ice cream try to reaching at the refrigerant temperature and melting point will be decreases and freezing point will be increases. For this particular reason the ice cream starts to change its state from liquid to solid.
Cooling of a hot rod:-
Cooling of a hot rod is another example of thermal expansion. When a rod has higher temperature after doing any operation it became hotter. When a hotter rod is placed in a normal room temperature that time the rod try to reach at the room temperature. The rod starts to decreases its temperature and became cool.
Making of tea:-
When we make tea that time with hot water milk is added at that time the temperature of milk is low and the temperature of water is cold but when hot water and cold milk is added to each the mixture comes in a normal temperature. So, making of tea is also another example of thermal expansion.
Frequent asked questions:-
Question: –
Write the formula for thermal equilibrium.
Solution: – When two different matters stay in same temperature that’s mean the two different matters maintain thermal equilibrium.
The formula for thermal equilibrium is,
Where,
Q = Total energy of the specific matter of the body which is expressed in Joule
m = Mass of the specific matter of the body which is expressed in grams
Ce = Specific heat of the specific matter of the body which is expressed in joule per Kelvin per kilogram
Δ t = (Final temperature – Starting temperature) of the specific matter of the body which is expressed in Kelvin
Question: –
In a house a bowl is present which is decorated with beautiful stones. The bowl is made with aluminium. The weight of the bowl is 15 gram and temperature is about 39 degree centigrade. Now in the aluminium bowl water is placed. At this condition the temperature of the water will be 20 degree centigrade and weight of the water is about 32 gram.
Find the exact temperature where the temperature of the aluminium and the temperature of the water will be same.
Solution: –
We know that,
Where,
Q = Total energy of the specific matter of the body
m = Mass of the specific matter of the body
Ce = Specific heat of the specific matter of the body
Δt= (Final temperature – Starting temperature) of the specific matter of the body
For aluminium,
Given data are,
mA = 15 gram
CeA = 0.215 calorie per gram degree centigrade
Δ tA = (Tf – TiA) degree centigrade = (Tf – 39) degree centigrade
For water,
QW = mW * CeW *ΔtW………….. eqn (1)
Given data are,
mW = 32 gram
CeW = 1 calorie per gram degree centigrade
ΔtW = (Tf – TiW) degree centigrade = (Tf – 20) degree centigrade
Now, from………….. eqn (1) and ………….. eqn (2) we can write,
Putting the value from eqn (1) and eqn (2),
(Put the value for CeW = 1 calorie per gram degree centigrade)
Tf= 21.7 degree centigrade
In a house a bowl is present which is decorated with beautiful stones. The bowl is made with aluminium. The weight of the bowl is 15 gram and temperature is about 39 degree centigrade. Now in the aluminium bowl water is placed. At this condition the temperature of the water will be 20 degree centigrade and weight of the water is about 32 gram
The exact temperature where the temperature of the aluminium and the temperature of the water will be same is 21.7 degree centigrade
Question: –
Explain types of thermodynamic equilibrium.
Solution: – A system is called thermodynamic equilibrium when mechanical equilibrium, thermal equilibrium and chemical equilibrium are same.
Thermodynamic equilibrium three types, they are,
Mechanical equilibrium:-
A system is called mechanical equilibrium when pressure will be no changed in any condition and also no changes in acting of unbalanced force.
Thermal equilibrium:-
A system is called thermal equilibrium when temperature will be no changed in any condition inside the system of the matter.
Chemical equilibrium:-
A system is called chemical equilibrium when chemical reaction no present inside the system and also any type of composition changes is not present of the matter.
- Why do materials have different stress thresholds? Exploring the factors
- How do materials recover after the removal of stress? Exploring the mechanisms of resilience
- Is Stress Always Harmful to Materials? Exploring the Impact and Resilience
- Does increasing cross-sectional area reduce stress? The science behind it
- When considering biomechanics, what role does stress play? Unveiling the Impact
- Why is understanding arterial wall stress essential in medical science? Exploring its impact on cardiovascular health