Hydrobromide acid reacts with the Epsom salt to form battery acid. Let us study HBr + MgSO4 reaction in depth.
Magnesium sulfate is a white crystalline solid, referred to as Epsom salt, a household chemical with different traditional uses. It is soluble in water and is mainly used in the agriculture field. Hydrogen bromide is a colorless gas, usually used as a reagent, in the production of bromide compounds.
This article studies the various fact of HBr + MgSO4 reactions like the enthalpy of the reaction, reaction type, product, ionic equation, conjugate pairs, etc.
What is the product of HBr + MgSO4?
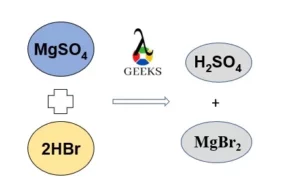
Hydrobromic acid (HBr) reacts with magnesium sulfate (MgSO4) to form sulphuric acid (H2SO4) and magnesium bromide (MgBr2).
HBr + MgSO4 → H2SO4 + MgBr2
What type of reaction is HBr + MgSO4?
HBr + MgSO4 is a double displacement, precipitate reaction, an exothermic reaction.
How to balance HBr + MgSO4?
The given HBr + MgSO4 chemical reaction is balanced by below steps:
- The unbalanced chemical reaction is written down as:
- MgSO4 + HBr → H2SO4 + MgBr2
- The number of moles of each element on either side of the arrow is tabulated as shown below:
| Elements | Reactant | Product |
|---|---|---|
| Mg | 1 | 1 |
| Br | 1 | 2 |
| S | 1 | 1 |
| O | 4 | 4 |
| H | 1 | 2 |
- The given chemical reaction is balanced when the number of moles of each element on the left side of the arrow is equal to the number of moles of each element on the right side of the arrow.
- Here moles of Br and H are unbalanced on either side.
- To balance the reaction, multiply 2 with HBr on the reactant side.
- The Balanced chemical reaction is
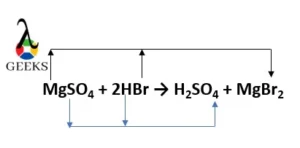
- MgSO4 + 2HBr → H2SO4 + MgBr2
HBr + MgSO4 Titration
HBr + MgSO4 titration is not practiced due to the formation of salt, that is MgBr2.
HBr + MgSO4 Net ionic equation
The net ionic equation for HBr + MgSO4 is not practiced because all ions are spectator ions in the reaction as shown below:
Mg2+(aq) + SO42-(aq) + 2H+(aq) + 2Br–(aq) = 2H+(aq) + SO42-(aq) + Mg2+(aq) + 2Br–(aq)
HBr + MgSO4 conjugate pairs
The conjugate acid-base pairs for HBr + MgSO4 are,
- Conjugate acid of HBr = HBr+
- Conjugate base of HBr = Br–
HBr + MgSO4 intermolecular forces
The intermolecular forces on HBr and MgSO4 are,
- Dipole-dipole interaction and London dispersion forces act on HBr
- Ion- Ion interactions, Dipole-dipole interaction, and London dispersion forces act on MgBr2
HBr + MgSO4 reaction enthalpy
The change in reaction enthalpy for HBr + MgSO4 is, -82.91 kJ/mol.
- The reaction enthalpy of formation of each compound on either side of the reaction is tabulated down as:
| Compounds | Enthalpy (kJ/mol) |
|---|---|
| MgSO4 | -1278.2 |
| HBr | -36.23 |
| H2SO4 | -909.27 |
| MgCl2 | -524.3 |
- Change in reaction enthalpy = Sum of enthalpies on the product side – the sum of enthalpies on the reactant side.
- Change in Enthalpy = (-909.27-524.3) – (-1278.2-36.23) = -82.91 kJ/mol
Is HBr + MgSO4 a buffer solution?
HBr + MgSO4 reaction is not a buffer solution since strong HBr acid is present which takes place during the reaction.
Is HBr + MgSO4 a complete reaction?
HBr + MgSO4 is defined as a complete reaction as at equilibrium complete moles of reactant are converted to form H2SO4 and MgBr2, no further reaction is possible.
Is HBr + MgSO4 an exothermic or endothermic reaction?
HBr + MgSO4 is an exothermic reaction because the change in enthalpy is -82.91 kJ/mol, which is negative thereby decreasing the temperature.
Is HBr + MgSO4 a redox reaction?
HBr + MgSO4 is not a redox reaction since there is no change in the oxidation state of elements observed.
Is HBr + MgSO4 a precipitation reaction?
HBr + MgSO4 is a precipitation reaction since the formation of MgBr2 takes place in the reaction which is a salt.
Is HBr + MgSO4 irreversible or irreversible reaction?
HBr + MgSO4 is an irreversible reaction, which can only be reversed unless and until there is a drastic change in experiment pressure or temperature.
Is HBr + MgSO4 displacement reaction?
HBr + MgSO4 reaction is a double displacement (metathesis) reaction. H2 is traded with SO4 to form H2SO4 and Br is traded with Mg to form MgBr2.
Conclusion
The reaction of magnesium sulfate with hydrobromic acid involves the formation of salt and sulfuric acid. This is an exothermic, precipitate reaction. Magnesium bromide is a deliquescent white crystalline solid, usually used with mild sensitive, and used as a catalyst in many other chemical reactions
Read more facts on HBr:
Hi….I am Pratham Manish Shah, Pursuing an Integrated MTech degree in Chemical Engineering from the Institute of Chemical Technology Mumbai Marathwada Jalna. With Lamdageeks, I am Interested in learning ongoing education opportunities to maintain knowledge of emerging technologies and methods.
Let’s connect via LinkedIn: www.linkedin.com/in/pratham-shah-07