Lithium(Li) is an s-block alkali metal and is extremely reactive in nature. Hydrogen bromide(HBr) is a strong inorganic acid. Let us examine the reaction between HBr and Li.
HBr is a strong acid with a pka value of -9. It is a corrosive, pale-yellow liquid with pungent odor. It is a good oxidizing agent and used in the production of organobromides. Li is a soft, silvery-white metal that loses its luster easily on exposure to air. Li is majorly used in the production of Li-ion batteries.
In this article, let us explore the product formed in the chemical reaction between HBr and Li, along with properties like the type of reaction, molecular forces, reaction enthalpy, etc.
What is the product of HBr and Li?
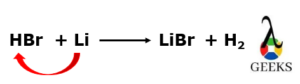
HBr reacts with Li metal to form lithium bromide (LiBr) with the liberation of hydrogen (H2) gas.
2HBr (aq) + 2Li (s) —–> 2LiBr (aq)+ H2 (g)
What type of reaction is HBr + Li?
The reaction of HBr + Li is an example of single displacement reaction.
How to balance HBr + Li?
The steps to balance HBr + Li are as follows.
2HBr + 2Li —–> 2LiBr + H2
- Determine the number of atoms present in both sides of the reaction.
| Elements | Reactant side | Product side |
|---|---|---|
| Li | 1 | 1 |
| Br | 1 | 1 |
| H | 1 | 2 |
- We find that there are unequal number of hydrogen atoms on either side of the reaction.
- To balance the number of H atoms, multiply HBr on the reactant side by 2, i.e., 2HBr + Li = LiBr + H2.
- To balance Br atoms on the product side in the above reaction, multiply LiBr by 2, i.e., 2HBr + Li = 2LiBr + H2.
- Finally, balance Li atoms on the reactant side by multiplying it by 2.
- Thus, the overall balanced reaction is given by
2HBr + 2Li —–> 2LiBr + H2
HBr + Li titration
HBr + Li titration does not occur, as lithium is an alkali metal.
HBr + Li net ionic equation
The net ionic equation between HBr + Li is
2H+ (aq) + 2Li (s) = 2Li+ (aq) + H2 (g)
The net ionic equation can be deduced by the following steps:
- Write the balanced equation along with the physical states of each molecule.
2HBr (aq) + 2Li (s) = 2LiBr (aq)+ H2 (g) - Write the ionic form of soluble strong electrolytes present in the reaction. Hence, the complete ionic equation is –
2H+ (aq) + 2Br– + 2Li (s) = 2Li+ (aq) + 2Br– (aq) + H2 (g) - In the final step, cancel the spectator ions (2Br–) on either side of the reaction to obtain the net ionic equation.
2H+ (aq) + 2Li(s) = 2Li+ (aq) + H2 (g)
HBr + Li conjugate pairs
- Conjugate base of strong acid HBr is Br–
- Li is a metal, so there is no conjugate pair for lithium.
HBr + Li intermolecular forces
- London dispersion forces, Vander Waal’s force of attraction and dipole-dipole interactive forces exist in HBr. The dipole-dipole interactions are predominant due to the greater electronegativity of bromine than the H-atom.
- Metallic bonding is seen in Li, as it is a metal.
HBr + Li reaction enthalpy
HBr + Li standard reaction enthalpy is -315.64 KJ/mol. The enthalpy of formation values is listed below: –
| Reactants and Products | Enthalpy in KJ/mol |
|---|---|
| HBr | -35.66 |
| Li | 0.0 |
| LiBr | -351.2 |
| H2 | 0.0 |
- The enthalpy of formation of elements and molecules existing in their natural state is taken as zero.
∆Hf°(reaction) = ∆Hf°(products) – ∆Hf°(reactants)
= -351.2 – (-35.66)
= -315.64 KJ/mol
Is HBr + Li a buffer solution?
HBr + Li does not form a buffer solution because HBr is a strong acid, and the salt of its conjugate base does not contain Li.
Is HBr + Li a complete reaction?
HBr + Li is a complete reaction, as it involves the formation of stable neutral lithium bromide salt and H2 gas.
Is HBr + Li an exothermic reaction?
HBr + Li is an exothermic reaction, as the reaction enthalpy is negative, and sufficient heat is generated along with the evolution of H2 gas.
Is HBr + Li a redox reaction?
HBr +Li is a redox reaction, as transfer of electrons occurs during the reaction, leading to simultaneous increase and decrease of oxidation number.
- Lithium gains an electron and is oxidized from 0 to +1 oxidation state.
- Hydrogen loses an electron and is reduced from +1 to 0 oxidation state.
Is HBr + Li a precipitation reaction?
HBr + Li is not a precipitation reaction as the product (LiBr) formed during the chemical reaction is a neutral salt, soluble in water and ethanol.
Is HBr + Li an irreversible reaction?
HBr + Li is an irreversible reaction since the reaction entropy rapidly increases due to the evolution of H2 gas, thereby increasing the feasibility of forward reaction.
Is HBr + Li a displacement reaction?
HBr + Li is an example of a single displacement reaction because only one of the chemical species, viz., hydrogen, is displaced from HBr by the more reactive Li, to form lithium bromide.
Conclusion
Lithium being an alkali metal, readily loses an electron and gets oxidized to LiBr on reaction with HBr. LiBr is a white hygroscopic water-soluble solid and is used as a desiccant in air-conditioning units.
Read more facts on HBr:

Hi Everyone. I am Vishnupriya T. I have pursued a Master’s in Chemistry and a PG Diploma in Quality Management. Currently working as an SME in Chemistry, aiming to enrich my curiosity and knowledge. I would love to share knowledge and connect with others at my LinkedIn profile: