Potassium sulphide (K2S) and hydrogen bromide (HBr) are inorganic base and acid respectively. Let us examine more about the reaction between HBr and K2S.
HBr is a strong acid with an easily ionizable covalent bond, due to the high electronegativity difference between H and Br atoms. It is a pale-yellow liquid useful in the production of organic bromides. K2S is a weak base and is deliquescent in nature. It is widely used as a reducing agent in analytical chemistry.
In this article, let us discuss a few facts based on the reaction between HBr and K2S, like the product formed, molecular forces, the type of reaction, buffer solution, etc.
What is the product of HBr and K2S?
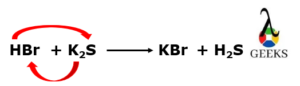
HBr reacts with K2S to form potassium bromide (KBr) and hydrogen sulphide (H2S) gas.
2HBr + K2S —–>2KBr + H2S
What type of reaction is HBr + K2S?
The reaction of hydrobromic acid and potassium sulphide is an acid-base reaction.
How to balance HBr + K2S?
The steps to balance HBr + K2S are as follows –
2HBr + K2S —–> 2KBr + H2S
- Determine the number of atoms involved in both sides of the reaction.
| Elements | Reactant side | Product side |
|---|---|---|
| K | 2 | 1 |
| S | 1 | 1 |
| Br | 1 | 1 |
| H | 1 | 2 |
- We find that the product side contains 2 atoms of Hydrogen. So to get an equal number of H atoms, multiply HBr by 2.
- Multiply KBr on the product side by 2, to equalize the number of K atoms on both sides of the equation.
- Hence, the overall balanced reaction is given by –
2HBr + K2S —–> 2KBr + H2S
HBr + K2S titration
HBr+ K2S titration is a strong acid versus weak base titration. In this reaction, our objective is to calculate the volume of potassium bromide formed during the reaction.
Apparatus
Burette, burette holder, pipette, distilled water, conical flask, round bottom flask, wash bottle, and beakers.
Indicator
Methyl orange is the acid-base indicator used in this reaction to detect the end point of the titration.
Procedure
- The burette is filled with standardized HBr solution.
- K2S is taken in a clean conical flask.
- 1-2 drops of methyl orange indicator is added. The solution in the conical flask turns yellow.
- HBr is added dropwise into the conical flask with continuous shaking until the yellow color just turns red. The change in color marks the end point of the titration.
- Note the volume of HBr used for the titration.
- The above procedure is repeated for minimum 3 concordant readings.
- The volume of KBr is calculated using the formula, SHBr VHBr = SK2S VK2S
HBr + K2S net ionic equation
The net ionic equation between HBr+ K2S is –
2H+ (aq) + S2- (aq) = H2S (g)
The net ionic equation can be derived by the following steps:
- Write the balanced equation along with the physical state of the molecules.
2HBr (aq) + K2S (aq) = 2KBr (aq)+ H2S (g) - Write the ionic form of the strong electrolytes existing in the aqueous form in the equation. Hence, the complete ionic equation is –
2H+ (aq) + 2Br– + 2K+ + S2- (aq) = 2K+ + 2Br– + H2S (g) - Cancel the spectator ions (2K+ and 2Br–) on either sides of the reaction, to get the net ionic equation.
2H+ (aq) + S2- (aq) = H2S (g)
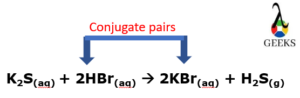
HBr + K2S conjugate pairs
HBr and Br– are the conjugate acid-base pairs in this reaction of HBr+K2S.
HBr + K2S intermolecular forces
- The intermolecular forces in HBr are dipole-dipole interactions and London dispersion forces, but dipole-dipole interactions are strong due to the high electronegativity of bromine.
- Ion-ion interactions exist in K2S molecule as an ionic bond is formed between potassium metal and sulfur, which is a non-metal.
HBr + K2S reaction enthalpy
HBr + K2S standard reaction enthalpy is -331.08 KJ/mol. The values of enthalpy of formation are listed below.
| Reactants and Products | Enthalpy in KJ/mol |
|---|---|
| HBr | -35.66 |
| K2S | -406.2 |
| KBr | -394 |
| H2S | -20.6 |
- ∆Hf°(reaction) = ∆Hf°(products) – ∆Hf°(reactants)
= -808.6 – (-477.52)
= -331.08 KJ/mol
Is HBr + K2S a buffer solution?
HBr + K2S is not a buffer solution as HBr is a strong acid, and a strong acid cannot be a part of a buffer solution.
Is HBr + K2S a complete reaction?
HBr + K2S is a complete reaction, as the product potassium bromide is soluble in water, so it does not react further.
Is HBr + K2S an exothermic reaction?
HBr + K2S is an exothermic reaction, as the reaction enthalpy is negative, and the heat generated is sufficient for the reaction to occur.
Is HBr + K2S a redox reaction?
HBr + K2S is not a redox reaction, as there is no transfer of electrons.
Is HBr + K2S a precipitation reaction?
HBr + K2S is not a precipitation reaction as the major product (KBr) formed is water soluble.
Is HBr + K2S an irreversible reaction?
HBr + K2S is an irreversible reaction because the reaction entropy increases due to the liberation of H2S gas, thereby favoring forward reaction.
Is HBr + K2S a displacement reaction?
The reaction between HBr + K2S is a double displacement reaction, because the anions and cations of the reactants swap places to form KBr and H2S.
Conclusion
The reactivity of HBr with K2S is more when compared to HCl, as HBr is a stronger acid. Precautions must be taken while handling K2S as it catches fire on exposure to air.
Read more facts on HBr:

Hi Everyone. I am Vishnupriya T. I have pursued a Master’s in Chemistry and a PG Diploma in Quality Management. Currently working as an SME in Chemistry, aiming to enrich my curiosity and knowledge. I would love to share knowledge and connect with others at my LinkedIn profile: