HBr is a very strong mineral acid and Aluminium is a soft silvery-white p-block element of group 13 of the periodic table. Let us learn about their reaction.
HBr is also known as Hydrobromic acid or Bromane. Aqueous solution of HBr appears to be a colourless or pale yellowish liquid and it completely dissociates in aqueous media to give H+ and Br–ion. Al is a soft, mouldable, light, ductile and non-corrosive metal that mainly exists in the +3 oxidation state.
Now, this article will cover some detailed discussions over the reaction between HBr and Al.
What is the product of HBr and Al?
The product formed by HBr +Al reaction is AlBr3 and H2 gas.
- 2Al(s) + 6HBr(aq) = 2AlBr3(s) + 3H2(g)
What type of reaction is HBr + Al?
HBr + Al is a single displacement type reaction.
How to balance HBr + Al?
To balance the HBr + Al reaction, the following steps are to be followed
- Write the reactants and products of the reaction.
- HBr + Al → AlBr3 + H2
- Count the number of atoms present on the reactant side and on the product side.
| Elements involved | Reactants side | Products side |
| H | 1 | 2 |
| Br | 1 | 3 |
| Al | 1 | 1 |
- Multiply with notable numbers to the specific reactants and products such that the number of each atom on the reactant side and the product side becomes equal.
- 6 × HBr and 2 × Al on reactant side and 3×H2 and 2×AlBr3 on the product side.
- Count the number of atoms after multiplication process to ensure equal number of atoms in both sides.
| Elements involved | Reactants side | Products side |
| H | 6 | 6 |
| Br | 6 | 6 |
| Al | 2 | 2 |
- Now write the complete balanced chemical equation.
- 6HBr + 2Al = 2AlBr3 + 3H2
HBr + Al titration
Titration is not possible because HBr +Al reaction produces a yellowish-white precipitate of AlBr3, and the reaction is highly exothermic, so difficult to operate.
HBr + Al net ionic equation
The net ionic equation of HBr +Al reaction is
6H+(aq) + 2Al(s) → 2Al3+(aq) + 3H2(g)
The equation is obtained by using the steps below
- Write the balanced chemical reaction
- 6HBr + 2Al = 2AlBr3 + 3H2
- HBr dissociates in aqueous medium as H+ and Br–.
- AlBr3 is polar covalent and dissociates as Al3+ and 3Br–.
- So the complete ionic equation is
- 6H+(aq) + 6Br–(aq) + 2Al → 2Al3+(aq) + 6Br–(aq) + 3H2(g)
- 6 Br- will be removed from both side to get the net ionic equation.
- 6H+(aq) + 2Al(s) → 2Al3+(aq) + 3H2(g)
HBr + Al conjugate pairs
- HBr loses a proton to give Br-, so Br- is the conjugate base of HBr.
- Al is a metal so does not behave as conjugate pair.
- AlBr3 is a Lewis acid but does not bear a proton, so don’t have conjugate base.
HBr and Al intermolecular forces
The intermolecular forces involved in HBr +Al reaction are:
- Due to high electronegativity difference between H and Br, It is strongly polar and possesses dipole-dipole interaction.
- AlBr3 is nonpolar and have covalent interaction.
- Al have metallic bonding character.
HBr + Al reaction enthalpy
The enthalpy change for the reaction HBr + Al is = -926.3KJ/mol
| Compounds | Number of Moles | Enthalpy of formation, ΔH0f (KJ/mol) |
| HBr | 6 | -36.45 KJ/mol |
| Al | 2 | 0 |
| AlBr3 | 2 | -572.5 KJ/mol |
| H2 | 3 | 0 |
- Reaction enthalpy =ΔH0f (reaction) = ΣΔH0f (product) – ΣΔH0f (reactants)
- ΔH0f (reaction)= [2×(-572.5) + 3×(0)] – [6×(-36.45) + 2×(0)] KJ/mol= -926.3 KJ/mol.
Is HBr + Al a buffer solution?
HBr + Al reaction is not a buffer solution as HBr is a strong mineral acid and dissociates to H+ and Br– in an aqueous medium.
Is HBr + Al a complete reaction?
It is a complete reaction as the products formed will not undergo further reaction.
6HBr + 2Al → 2AlBr3 + 3H2 (g)
Is HBr + Al an exothermic or endothermic reaction?
The HBr + Al reaction is an exothermic reaction as the enthalpy change is negative and 926.3 KJ /mol energy is released in form of heat.
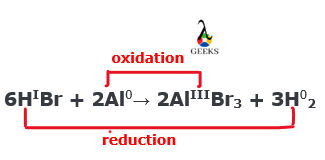
Is HBr + Al a redox reaction?
The HBr + Al reaction is a redox reaction. Here Al is oxidized from 0 to +3 oxidation state, and H is reduced from +1 to 0 oxidation state.
Is HBr + Al a precipitation reaction?
HBr + Al reaction is a precipitation reaction because it gives a white or faint yellowish precipitate of AlBr3 in the reaction mixture and H2 gas is released.
Is HBr + Al a reversible or irreversible reaction?
HBr + Al reaction is irreversible as the forward response is more favorable thermodynamically due to increase in entropy by the release of gaseous product H2(g).
Is HBr + Al displacement reaction?
HBr + Al reaction is a displacement reaction as , the cation Al3+ displaces the H+ ion of HBr to form the products AlBr3 and H2 gas.
Conclusion
The reaction of HBr and Al is exothermic and takes place at room temperature. HBr has widespread use in organic synthesis and Al is an extremely useful metal. Aluminium bromide sometimes used as a catalyst in Friedel-Crafts alkylation.
Read more facts on HBr:

Hello….I am Soumak Mahato. I have completed my M.Sc in Chemistry from Banaras Hindu University in 2022, specializing in Inorganic Chemistry. I have joined Lambdageeks as an SME in Chemistry. Trying to explain chemistry in easy way. My hobbies include Sports and music.
Let’s connect through LinkedIn