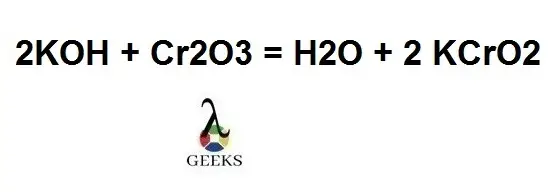Inorganic reaction (KOH + Cr2O3) produces KCrO2 appears as powder porm.
In this article we see reaction of various components with Cr2O3 in basic medium, KOH where Cr2O3 acts like a reducing agent.
What is Cr2o3 + Koh?
At a certain pressure and temperature Chromium (III) oxide and Potassium hydroxide react and as product we get KCrO2 and Water (H2O), where the oxidation state remains same for Chromium (Cr) which is (3+).
What is the product of Cr2o3 + Koh?
In the reaction, KCrO2 and water (H2O) produced after Cr2O3 and KOH react, if we balance the reaction by both reactant and product sides we get, 2KOH + Cr2O3 = H2O + 2 KCrO2, where the oxidation state of Chromium (Cr) is (3+).

How to balance Cr2o3 + Koh?
If we balance a reaction we can find how many molecules are required to produce the product, to do this we lable each of the component of the reaction by a, b, c, d etc as coefficients, say like for a KOH + b Cr2O3 = c H2O + d KCrO2.
Then create equations with the help of the coefficients as the number of components are matched like; (1) a = d for ‘K’, (2) 2b = d for ‘Cr’, (3) a + 3b = c + 2d for Oxygen and (4) a = 2c for Hydrogen. After that by solving the solutions we get the numbers.
Here a = d = 2b = 2c, so if we consider a and d as 2 then b and c become, and we get the coefficients of the components of the reaction.
What type of reaction is Cr2o3 + Koh?
The chemical reaction of CrO3 + KOH is a neutralization reaction as the reactant medium is base where chromium oxide (CrO3) reacts with basic KOH producing KCrO2 with water molecule (H2O).
Cr2o3+Koh+H2o
In this reaction Potassium hexahydroxochromate(III) is produced which is a complex type of molecule, by balancing the reactant and product side we get, Cr2O3 + 6 KOH + 3 H2O = 2 K3[Cr(OH)6].
If we check the oxidation state for Chromium (Cr) in the reactant side and in the product side it changes from (3+) to (6+), the oxidation state increases means Cr release more three electrons so it act as a reducing agent.
In the product [Cr(OH)6](3-) is the coordination entity where Cr(III) is the central atom as the ligands 6 (-OH) are attached with it, so the coordination number will be 6 as those number of ligands are attached with the central atom.
cr2o3+koh+kclo3
In the reaction Potassium chromate (K2CrO4), Potassium chloride (KCl) and water (H2O) are formed, where balancing the reaction we get, KClO3 (aq) + Cr2O3 (s) + 4 KOH (aq) = KCl (aq) + 2 K2CrO4 (aq) + 2 H2O (l).
If we check the oxidation states of Chromium (Cr) and Chlorine (Cl), we found that they change from reactant side to product side, as Cr2O3 transfer to K2CrO4 where oxidation state of Chromium increases from (+3) to (+6) as two Cr(+3) release six electrons.
So Cr2O3 is a reducing agent in the reaction as these releasing six electrons are accepted by Chlorine of KClO3 and changes its oxidation state from (+5) to (-1), so KClO3 acts as an oxidizing agent here.

Cr2o3+Koh+Kno3
In the reaction Potassium nitrite (KNO2), Potassium chromate (K2CrO4) and water (H2O) are formed by balancing the reaction both in reactant and product side, we get, 3 KNO3 (aq) + Cr2O3 (s) + 4 KOH (aq) = 3 KNO2 (aq) + 2 K2CrO4 (aq) + 2 H2O (l).
In the redox reaction, the reduction reaction occur when KNO3 is converted to KNO2, where the oxidation state of Nitrogen atom transfer from (+5) to (+3) by accepting total 6 electrons for three Nitrogen atoms from Cr (III), so KNO3 is an oxidizing agent.
Simultaneously oxidation process (electron transferring process) also occurs where Cr2O3 act like reducing agent as Chromium increases its oxidation state from (+3) to (+6) when Cr2O3 transfer to K2CrO4, the releasing electrons reduce Nitrogen of KNO3.
cr2o3+koh+cl2
In the reaction Potassium chloride (KCl), Potassium chromate (K2CrO4) and water (H2O) are formed by balancing both reactant and product side, we get, 3 Cl2 (g) + Cr2O3 (s) + 10 KOH (aq) = 2 K2CrO4 (aq) + 5 H2O (l).
In oxidation process where electron transfers from an atomic orbital to acceptor occurs, Cr2O3 act like reducing agent as Chromium changes its oxidation state from (+3) to (+6) by transferring electrons when Cr2O3 transfer to Potassium chromate.
In the reduction process where atomic vacant orbital accept electrons, Cl2 act as oxidizing agent as Chlorine decreases its oxidation state from zero (0) to (-1) by accepting electrons when 3 Chlorine (Cl) molecules form 6 Potassium chloride molecules.
cr2o3+koh+ca(clo)2
In the redox reaction Potassium chromate (K2CrO4), Calcium chloride (CaCl2) and water (H2O) are formed, where balancing both side of the reaction we get, 3 Ca(ClO)2 (s) + 2 Cr2O3 (s) + 8 KOH (aq) = 3 CaCl2 (aq) + 4 K2CrO4 (aq) + 4 H2O (l).
If we check the oxidation states of Chromium (Cr) and Chlorine (Cl) we found that they change from reactant side to product side, as Chromium (III) oxide transfer to K2CrO4 where oxidation state of Chromium changes from (+3) to (+6), is a oxidation reaction.
So Cr2O3 is act like reducing agent in the reaction as these releasing six electrons are accepted by Chlorine atom of three Ca(ClO)2 and changes its oxidation state from (+1) to (-1), so Ca(ClO)2 acts as an oxidizing agent here by accepting the electrons from Cr(III).
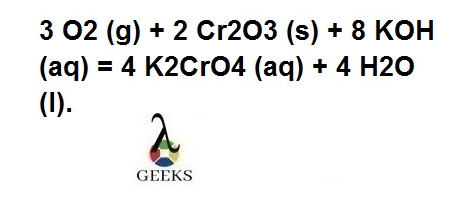
cr2o3+koh+o2
In the reaction Potassium chromate (K2CrO4) and water (H2O) are formed by both reactant and product side balancing, we get, 3 O2 (g) + 2 Cr2O3 (s) + 8 KOH (aq) = 4 K2CrO4 (aq) + 4 H2O (l).
In oxidation process where Oxygen is added or electron transfers from an atom, Cr2O3 act like reducing agent as Chromium increases its oxidation state from (+3) to (+6) by transferring electrons when Cr2O3 transfer to K2CrO4.
In the reduction process where Hydrogen is added or atom accept electrons, O2 act as oxidizing agent as Oxygen decreases its oxidation state from zero (0) to (-2) by accepting electrons when 3 Oxygen molecules form 4 water (H2O) molecules.
cr2o3+koh+h2o2
In the reaction Potassium chromate (K2CrO4) and water (H2O) are formed where both reactant and product side balancing, we get, 3 H2O2 (aq) + Cr2O3 (s) + 4 KOH (aq) = 2 K2CrO4 (aq) + 5 H2O (l).
In oxidation process where Oxygen is added to an atom, Cr2O3 act like reducing agent as Chromium increases its oxidation state from (+3) to (+6) by transferring electrons when Cr2O3 transfer to K2CrO4, where reduction reaction also occurs simultaneously.
In the reduction process where Hydrogen is added to atom or Oxygen is removed from an atom, H2O2 act as oxidizing agent as Oxygen decreases its oxidation state from (-1) to (-2) by accepting electrons when Hydrogen peroxide molecules form water (H2O) molecules.
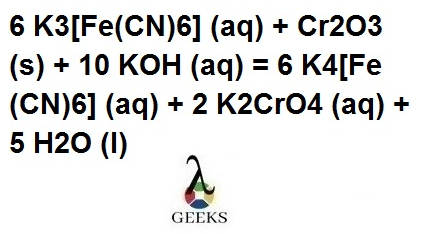
k3fe(cn)6 + cr2o3 + koh
In the reaction,a complex compound, K4[Fe(CN)6], Potassium chromate (K2CrO4), and water (H2O) are formed, where balancing the reaction we get, 6 K3[Fe(CN)6] (aq) + Cr2O3 (s) + 10 KOH (aq) = 6 K4[Fe(CN)6] (aq) + 2 K2CrO4 (aq) + 5 H2O (l).
If we check the oxidation states of Chromium (Cr) and Iron (Fe), we found that they change from reactant side to product side, as Cr2O3 produces K2CrO4 where oxidation state of Chromium (Cr) increases from (+3) to (+6) as Cr(+3) release three more electrons.
So Cr2O3 is a reducing agent in the reaction as these releasing six electrons are accepted by six Fe (III) of the complex compound and changes its oxidation state from (+3) to (+2), so K3[Fe(CN)6] acts as an oxidizing agent here by accepting electron.
kclo4+cr2o3+koh
In the reaction Potassium chromate (K2CrO4), Potassium chloride (KCl) and water (H2O) are formed, where balancing the reaction we get, 3 KClO4 (aq) + 4 Cr2O3 (s) + 16 KOH (aq) = 3 KCl (aq) + 8 K2CrO4 (aq) + 8 H2O (l).
If we check the oxidation states of reactant side comparing with product side of Chromium (Cr) and Chlorine (Cl), we found they change, as 4 Cr2O3 transfer to 8 K2CrO4 where oxidation state of Cr increases from (+3) to (+6) as two Cr(+3) release six electrons.
So Cr2O3 is a reducing agent in the reaction as these releasing six electrons which are transfer to orbital of Chlorine of KClO4 and changes its oxidation state from (+7) to (-1), form KCl molecule so KClO4 acts as an oxidizing agent here.
kclo+cr2o3+koh
In the redox reaction Potassium chromate (K2CrO4), Potassium chloride (KCl) and water (H2O) are formed, where balancing both side of the reaction we get, 3 KClO (l) + Cr2O3 (s) + 4 KOH (aq) = 3 KCl (aq) + 2 K2CrO4 (aq) + 2 H2O (l).
If we check the oxidation states of Chromium (Cr) and Chlorine (Cl), we found that they change from reactant side to product side, as Chromium (III) oxide transfer to K2CrO4 where oxidation state of Chromium increases from (+3) to (+6) as two Cr(+3) release six electrons.
So Cr2O3 is act like reducing agent in the reaction as these releasing six electrons are accepted by Chlorine of three KClO3 and changes its oxidation state from (+1) to (-1), so KClO acts as an oxidizing agent here by accepting the electrons.
nai+cr2o3+koh
After reacting with NaI and KOH, KI and NaOH are formed. KI can react with Cr2O3 in acid medium like H2SO4 but reaction is not observed in basic medium like NaOH.
kcl+cr2o3+koh
The reaction of Cr2O3 can be seen with potassium chlorate or Potassium perchlorate but not seen with potassium chloride.
cr2o3+nano3+koh
In the reaction Sodium nitrite (NaNO2), Potassium chromate (K2CrO4) and water (H2O) are formed by balancing the reaction we get, 3 NaNO3 (aq) + Cr2O3 (s) + 4 KOH (aq) = 3 NaNO2 (aq) + 2 K2CrO4 (aq) + 2 H2O (l).
In the redox reaction, the reduction reaction occur when NaNO3 is converted to NaNO2, where the oxidation state of Nitrogen atom transfer from (+5) to (+3) by accepting total 6 electrons for three Nitrogen atoms, so NaNO3 is an oxidizing agent.
Simultaneously oxidation process also occurs with reduction reaction where Cr2O3 transfer to K2CrO4 in which the oxidation state of Chromium increases from (+3) to (+6) as two Cr(+3) release six electrons ( three for each), so Cr2O3 is a reducing agent.
cr2o3+br2+koh
In the reaction Potassium bromide (KBr), Potassium chromate (K2CrO4) and water (H2O) is produced which is a redox reaction where oxidation and reduction of the reactant components form simultaneously.
By balancing both side of the reaction we get, Cr2O3 (s) + 3 Br2 (aq) + 10 KOH (aq) = 6 KBr (aq) + 2 K2CrO4 (aq) + 5 H2O (l), where (s) indicates solid molecule, (aq) indicates the component is in aqueous medium and (l) shows the state of component is liquid.
In reaction oxidation state of Br (oxidizing agent) changes from 0 to (-1), so Br2 to KBr formation is a reduction process where as oxidation state of Cr changes from (+3) to (+4) means it transfers one more electron which is an oxidation reaction, Cr2O3 is reducing agent.
Conclusion:
From the study of above reactions we can say that Cr2O3 is a good reducing agent, can donate electrons and show many oxidation states of Chromium (Cr) which is possible as Cr has ‘d’ orbital.