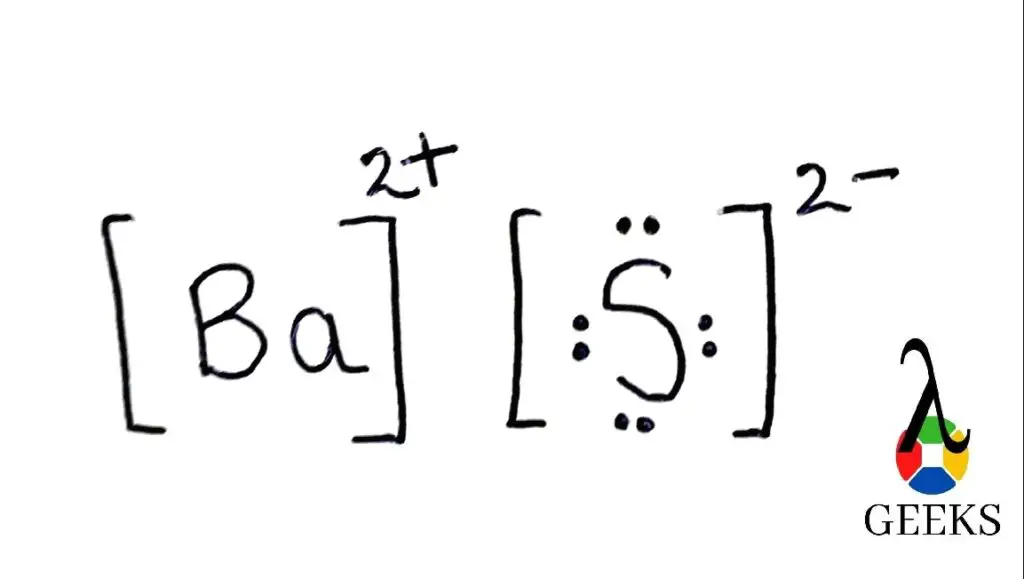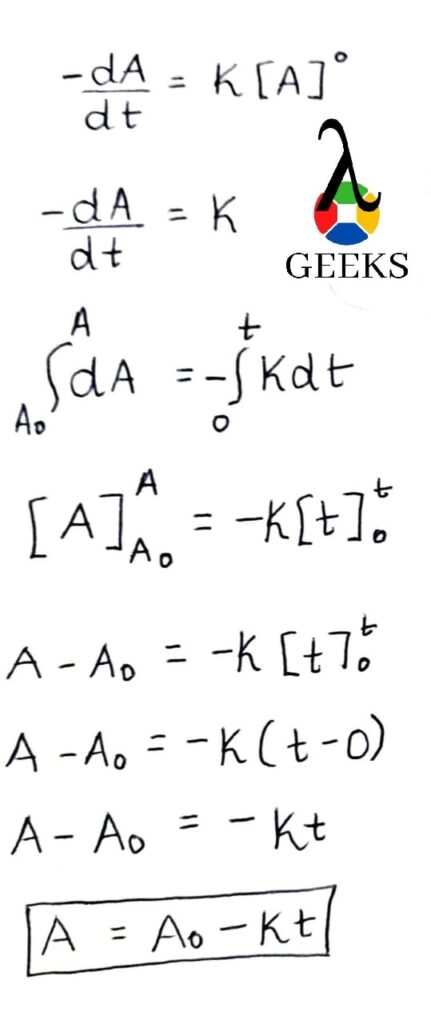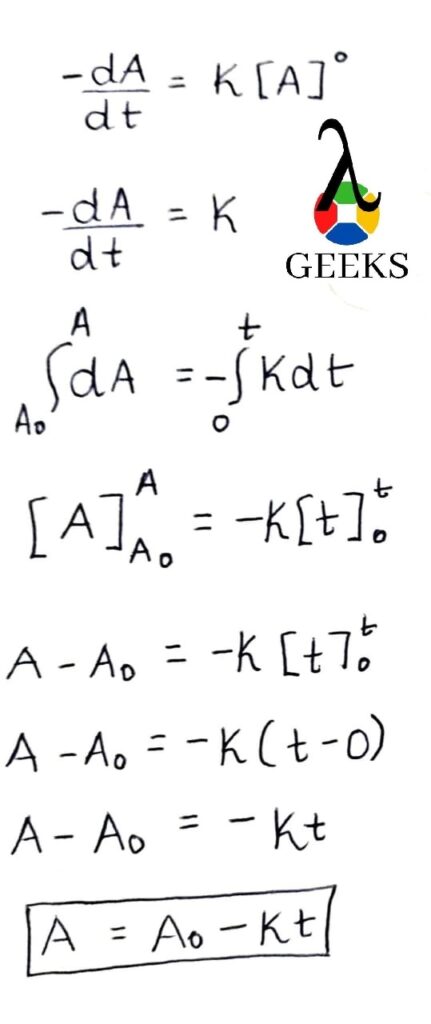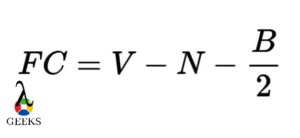In this article, we are going to study the Bas lewis structure and the important facts associated with it.
By using the lewis concept (bas Lewis structure) we can understand the bonding between atoms in a molecule. So we are going to apply this concept to barium sulfide and study the molecule in detail.
How to draw Bas lewis structure?
So in order to understand the bas Lewis structure we must know the number and types of atoms present in the molecule. There is one barium atom and one sulfur atom.
As we can see the bond formation is taking place between a metal (barium) and a non metal(sulfur). So the resultant compound will be of the ionic type. So in this type of bonding what will happen is valence electrons will be transferred to the non metal from the metal ( barium to sulfur). Valence electrons that are contributed by Ba is 2 and S is 6.
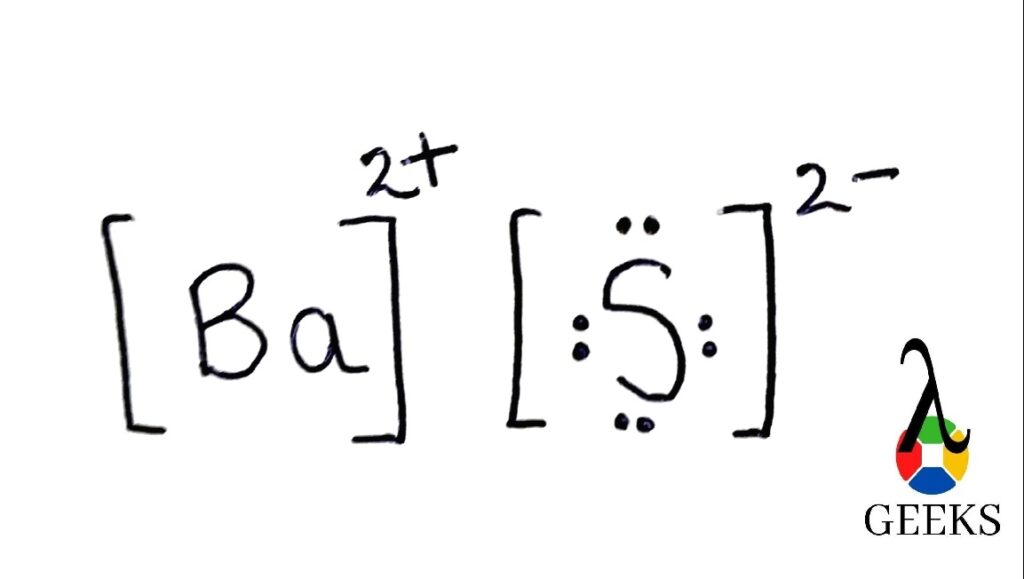
Hence the sum of all the Valence Electrons is 8 electrons. So in order to satisfy the octet of sulfur, barium will give 2 of its electrons to sulfur. In this way it will lead to the formation of ionic bond of barium sulfide.
Bas lewis structure resonance?
What we understand by resonating structure of a molecule are the structures(lewis) through which we can understand electrons delocalization in molecule or an ion(polyatomic).
The important condition for a molecule to resonate is it should possess at least one double bond and at least one pair of electrons that are available for donation.
So a lewis structure is of great help in studying the molecule.
Bas lewis structure shape
Barium sulfide is observed to be a colorless(crystalline type of solid). Having a density of around 4.25 g/cm3.
The observed melting point of this particular compound is around 2235 degrees Celsius and talking about its boiling point it decomposes very soon. So the shape of barium sulfide molecule is octahedral type. Where the cation has anions surrounding it.
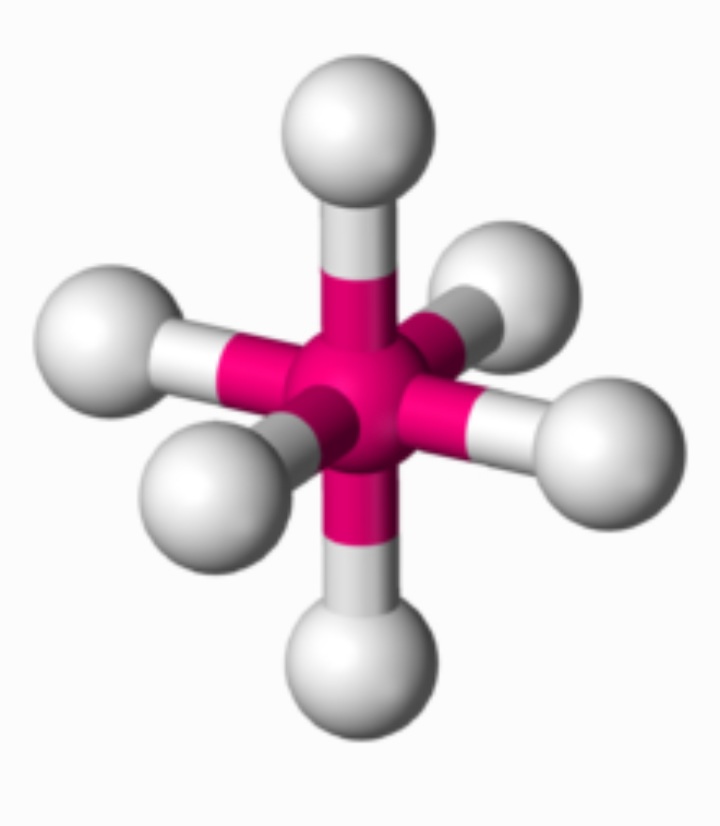
Bas lewis structure formal charge
We can carry out the calculation of formal charge by making use of the formula given below.
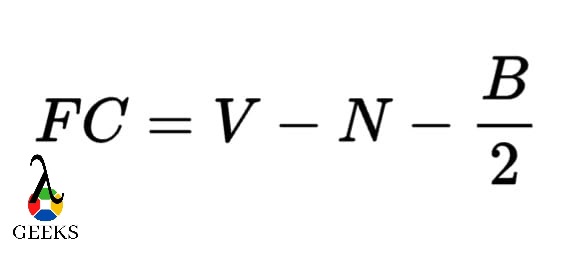
V symbolizes all the valence electrons that are present
N symbolizes all the non-bonding type of electrons(valence).
B symbolizes all the electrons that are shared in the bonds.
Hence the formal charge on the barium sulfide molecule is zero.
Bas lewis structure angle
We can that there are 2 atoms that participate in the formation of barium sulfide.
Out of which on is a barium element atom and the other is sulfur atom. As the compound is an example of ionic and the structure will be having octahedral type geometry. The bond angle of octahedral type of geometry is around 180 degrees.
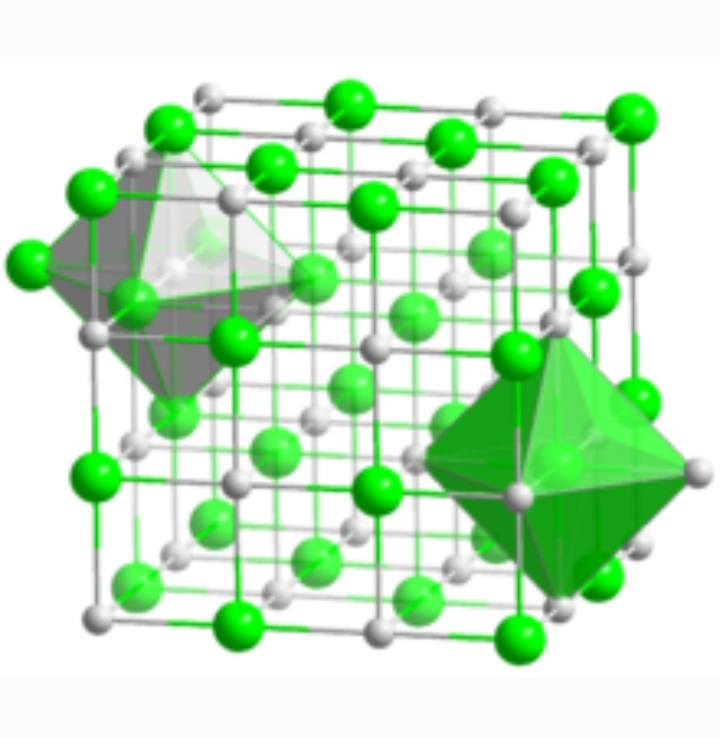
Bas lewis structure octet rule
In concept of octet rule, the atoms forming a molecule give away or accept electrons to satisfy their octet.
Meaning the outermost shell of an atom should be filled completely. This is called obtaining of a completely filled octet. So in the molecule of barium sulfide, barium gives away 2 of its available valence electrons to the sulfur atom (which has 6 valence electrons).
In this way an ionic bond is formed and the octet of barium sulfide is satisfied.
Bas lewis structure lone pairs
We know that a barium molecule has octahedral type of geometry. So contribution of valence type of electrons is 8. Presence of lone pairs has got very importance as it is responsible for predicting the structure of that particular molecule.
Bas valence electrons
The total valence electrons in the barium element are 2.
The total valence electrons in sulfur atoms are 6. So the total valence electrons present in the molecule will be 2+6=8. Hence two valence electrons of barium are donated to sulfur and its octet is satisfied.
Bas hybridization
Hybridization is the process where orbitals (atomic) are mixed so as to form new set of orbitals. This newly formed orbitals have totally different kind of shape, energy. If the s and p character is 50% each the it leads to sp hybridization.
Bas solubility
Coming to the solubility of barium sulfide, it will differ as we change the temperature.
When the temperature is zero degrees Celsius (less) the solubility is 2.8 g/100mL. When the temperature is 20 degrees Celsius (moderate) solubility is 7.68 g/100mL. When the temperature is 100 degrees Celsius (high) the solubility is 60.3 g/100mL.
It has been observed that barium sulfide will not dissolve in methanol and ethanol.
Is Bas ionic
A compound is said to be ionic when the bond formation occurs between a metal and a non metal. In the example of barium sulfide barium being a metal, sulfur (non metal), the bond is occurring between them. Thus the barium sulfide is an ionic type of compound.
Is Bas polar or non polar
A compound will be polar if there is at least some amount of difference in the electronegativity of atoms being involved for bond formation.
A non polar type of compound will not have any kind of difference w.r.t electronegativity. Meaning there is equal sharing of charge. The electronegativity of barium is 0.9 and the electronegativity of sulfur is 2.6. The electronegativity difference for the compound Bas is hence 1.77.
So yes there is some amount of difference in the electronegativity, hence the barium sulfide compound is polar.
Is Bas acidic or basic
Bas is an inorganic type of compound. So we can say barium sulfide is a source of sulfate and it is (moderately) soluble in acids and H2O.
Conclusion
To wrap with the post we can say that barium sulfide is an inorganic type of compound which has octahedral kind of geometry. The bond formation in the molecule takes place by the metal giving 2 electrons to the non metal and the resulting is an ionic kind of compound.
Also Read: