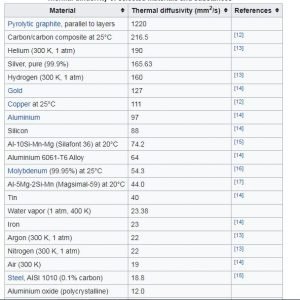
Content
Key notes
what is a hydronic heating system?
The hydronic system is used to warm your home. The water is used as a working fluid in most of the system. First, it will get heated by a boiler or other heating sources. Then, the water will be circulated through the combustion chamber via a heat exchanger.
Hydronic radiant floor heating system
The heated water is passed through the tubes inside the radiator on the floor. The floor is constructed such that it contains some holes like porous material. It may be wood or tiles with porous holes in them. The radiated heat is circulated inside the home. The use of carpet is avoided on the floor due to its low conductivity of heat.
hydronic heating system diagram Schematic
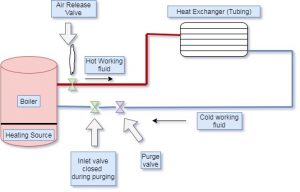
How to bleed air from hydronic heating system ?
- First of all, finds and identifies the air bleed valve near to radiator. This valve is small and cylindrical with one inch in height. The head of this valve is a slotted screw with a small nozzle inside it.
- After finding the air bleed valve, turn the air bleed valve in an anti-clockwise direction to open it. If air is present in the heating system, the air will come out with the valve opening through the nozzle. The water particles also come out with air. Keep valve open for some time till the complete water starts coming out without air. Close the valve once you notice the steady water flow.
- Closing of the valve can be done by rotating the air valve in a clockwise direction. In some heating systems, there are multiple valves installed in the system. In present case, we have to repeat this steps for all air valve
hydronic baseboard heating system
In Hydronic baseboard heating system, the heater will heat the liquid inside the system. The liquid should possess the non-toxicity. It may be water or some special type of oil. It should also give radiant heat to warm our house inside.
This type of system is similar to working of radiator, but the difference is that it possesses less space area to compare to the radiator.
hydronic radiant floor heating system | hydronic floor heating system | hydronic radiant heating systems
The radiant floor heating system is used to heat the area by using infrared radiations. It provides warm comfort to people inside the room. Compared with another heating air method, a radiant floor heating system is more efficient and convenient. In addition, the heat flows from ground to up so that the temperature is more maintained with a little cold.
In this kind of system, the heat will get radiated from the floor. This may be more beneficial for getting more convenient heat. We can feel more similar like one feels heat from stove burner at some distance from the stove. In this system, the air is not directly heated; the heat is radiated from ground level. It makes more warming effect and comfort to one using this system.
In a radiant floor heating system, the working fluid used is water. This water will get heated from outside heating sources like a water heater, geothermal or boiler, etc. The water is circulated through the PEX tubes, which are installed inside the home. It might be considered as a dry install or wet install
what is a hydronic heating system ? | hydronic water heating system | aqua hot hydronic heating system | how does a hydronic heating system work | basic hydronic heating system
The hydronic system is used to warm your home. The water is used as a working fluid in most of the system. First, it will get heated by a boiler or other heating sources. Then, the water will be circulated through the combustion chamber via a heat exchanger. Once the water absorbs the heat from the boiler, it will pass through the baseboard or radiator to rejects its heat. Finally, the baseboard or radiator is installed inside your home. This is a cyclic process of water to get heat from the heating source and reject heat inside a house from the baseboard.
Safety is provided in the system to avoid getting damage. For example, if the water level decreases in the baseboard, it will automatically shut off the boiler’s working to prevent an accident.
There is some indirect heating system work on two in one principle. The heat is utilized for warming homes as well as stored in a tank for other purposes.
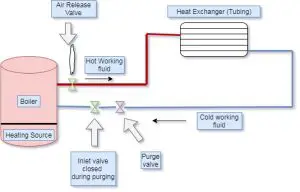
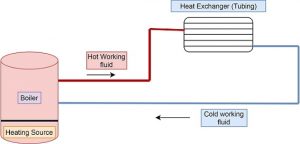
hydronic heating system diagram Schematic | residential hydronic heating system diagram schematic | hydronic heating system schematic | boiler hydronic heating system diagram
The schematic diagram of present system is shown in figure below,
types of hydronic heating systems in detail | hot water hydronic heating systems boilers | electric hydronic baseboard heating systems | boiler hydronic heating system
There are widely known three hydronic systems as expressed as below,
Hydronic radiant floor heating system :
In this heating system, the floor is covered with a huge radiator. The heated water is passed through the tubes inside the radiator on the floor. The floor is constructed such that it contains some holes like porous material. It may be wood or tiles with porous holes in them. The radiated heat is circulated inside the home. The use of carpet is avoided on the floor due to its low conductivity of heat.
Baseboard :
This system is also known as a “hot water baseboard heating system.” This system is a attractive due to its efficient working.
The hot water tubes with fins are kept inside the steel housing of the baseboard. The fins are useful to radiate heat from the pipe. The hot water is circulated through tubes.
Hydro air heating system :
Hydro air heating system includes duct and air handler unit. The hot working fluid is passed through the heat exchanger built in the air handler unit. The air will get heated with an air handler heat exchanger and distributed to the home. This system is less costly as compared to the radiant floor heating system. In addition, this system includes a duct, which can also be useful in an air conditioning system.
hydronic heating system components | hydronic heating system parts | expansion tank hydronic heating system | hydronic heating system expansion tank | components of hydronic heating system
The components used in hydronic systems are explained below. The main outside element of this system is the heating source. It may be a boiler, geothermal, water heater, etc.
Expansion tank:
The expansion tank is utilized to keep the excess working fluid passing through the system. The volume of working fluid is raised when it will get warm. To accommodate this volume, the expansion tank is used in this heating system. There is mainly two types of expansion tank are used in this system: either compression tank or standard basic tank.
Centrifugal pump :
This is also the main component of the system. It is used to circulate water throughout the system from the heating source to the home (heat exchanger). The centrifugal pump continuously runs to obtain the cyclic process of the system. The impeller is mounted on a shaft that pressurize the water to get circulate through the system. To avoid corrosion in the pump, the impellers are made of anticorrosive materials like bronze.
Air separator:
It is required to separate the air which is trapped in water. The air separator is the device that prevents air from getting trapped. The water will get pass through an air separator. The air separator is constricted with a wire screen which separates the air bubbles from the water. The trapped bubbles will get removed from the air vent. The separation of air in this system is necessary to avoid corrosion of metal and compressibility effect.
Air vent :
It is used to take out the air from the system. This device is installed with an air separator. It is preferred to use an automatic air vent device because the opening and closing of the device are very convenient. It is available in automatic as well manual mode.
hydronic forced-air heating system
Hydro air heating system :
Hydro air heating system includes duct and air handler unit. The heated working fluid is passed through the heat exchanger unit kept in the air handler. The air will get heated with an air handler heat exchanger and distributed to the home. This system is less costly as compared to the radiant floor heating system. This system includes a duct which can also be useful in an air conditioning system.
open-loop hydronic heating system | tankless hydronic heating system | open hydronic heating system
In an open-loop hydronic system, the working water in the system will get mixed with hot drinking water. The system is the unique for both working fluid
hydronic heating system operating pressure | closed loop hydronic heating system pressure
The operating pressure in a hydronic heating system is around 12 to 15 PSI (Pound per square inches). The pressure is enough to fill the water through the entire piping circuit. There are variations in operating pressure according to the various hydronic heating system and their components. This pressure range also depends on the range of centrifugal pumps used for water circulation through the system.
closed hydronic heating system
In a closed-loop heating system, the loop of the PEX tube is used as a heat exchanger with compactness. The connection of this tubing is with heat pumps and indoor units. The antifreeze solution is added to the working fluid to prevent it from freezing. This working fluid circulated through the complete system in a cyclic process. This system is reliable and economical if it is perfectly installed.
How to bleed air from hydronic heating system | purge air from the hydronic heating system with circulators | hydronic heating system air vent | hydronic heating system air eliminator
The water contains dissolved air with water molecules. In a hydronic system, the temperature of working fluid is raised at certain temperature. The heating of water separates the air from water. Therefore, it is required to take out this air from the system to avoid some losses. If these airs get trapped with hot water inside the tubes, it will damage the tube, generate noise and block the flow of hot water. In addition, the working efficiency of the total system will get decreased because of trapped air.
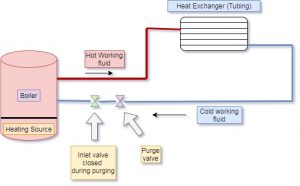
Generally, the air bleed valve is provided to take out this trapped air in the hydronic system. This air bleed valve is installed near to radiator.
The air bleeding from the heating system will follows the steps as given below,
- First of all, finds and identifies the air bleed valve near to radiator. This valve is small and cylindrical with one inch in height. The head of this valve is a slotted screw with a small nozzle inside it.
- After finding the air bleed valve, turn the air bleed valve in an anti-clockwise direction to open it. If air is present in the heating system, the air will come out with the valve opening through the nozzle. The water particles also come out with air. Keep valve open for some time till the complete water starts coming out without air. Close the valve once you notice the steady water flow.
- Closing of the valve can be done by rotating the air valve in a clockwise direction. In some heating systems, there are multiple valves installed in the system. In present case, we have to repeat this steps for all air valve
how does air get into a hydronic heating system
The air will get trapped in a hydronic heating system with many causes, let’s see some of the main causes as below,
- The air will get trapped when we are filling water into the system
- If we backflush the water from the hydronic heating system
- The water contains dissolved air with water molecules. In a hydronic heating system, the temperature of working fluid will get raised at a certain temperature. The heating of water separates the air from water.
- It will get trapped if any leakage in the heating system
how to flush a hydronic heating system | hydronic heating system flush | flushing hydronic heating systems | how to drain hydronic heating system
The method of flushing or draining for the hydronic heating system is explained with steps as below,
- Switch off the heating device first, let working fluid get cool and safe
- Close the valve for the water supply
- Join the one end of the hose to the drainage valve of the boiler.
- Open the drain valve of the boiler, also open all air vent valve of the heat exchanger (radiator)
- Now, after completion of the drain, close all air vent valves and drain valves.
- Start filling system again to make system work again.
how to install hydronic in-floor heating systems | building a hydronic heating system | hydronic radiant floor heating systems design | how to install hydronic heating system | hydronic radiant heating system design
The installation of the hydronic in-floor heating system can be done with following probable steps,
Step 1: The system is designed properly to estimate required parts and tools for installation
Step 2: make a bed of concrete and provide insulation over it. This insulation prohibits heat get flow in the bottom of tubes.
Step 3: Make a proper arrangement of reinforcement wire and put a tube over it properly.
Step 4: Install all tubes properly as per heat exchanger standard
Step 5: make floor slab ready completely
Step 6: Before start working on the system, properly check for any leakages in the system
Step 7: Fill the slab from the top and cover it
Step 8: Now, the system is ready to start work. Make proper adjustments of valves and devices so that in between, you can operate it if needed.
Step 9: Start your system and enjoy a warm atmosphere inside your home.
Step 10: The system is working first time, so that check for any troubleshoot and solve the problem if any arising
Step 11: Enjoy Your Cozy House!
hydronic heating system problems | hydronic heating system maintenance
There are some problem occurs during the working of a hydronic heating system working and operation. It is pointed as below,
- Hydronic Heating basic faults
- Flushing: periodic flushing is necessary for any hydronic heating system. The power flushing is required to be done every ten years. All the system suppliers recommend it.
- Air trapping: Air trapping in the radiator is one of the main issues in any hydronic system which stops its works. The air is taken out from the system through air vent valves periodically.
- The cost of sludge and scale removal is too high for any hydronic system. In addition, the sludge and scale formation depends on the quality of water used as a working fluid.
- Failure of the circulation pump and its performance
- Boiler noise creates a noisy atmosphere and inconvenient
- Antifreeze agent adjustment
- The periodic cleaning is required for complete system
hydronic heating system temperature
the temperature order for two different hydronic systems is given as below; this data is probable
- The temperature range in the radiant floor heating system is around 30 to 60 degrees centigrade.
- The typical temperature range for the baseboard system is approximately 55 to 70 degrees celsius.
- According to data, at this temperature range, the life of the boiler is expected 45 years
how to fill hydronic heating system with antifreeze
The following are the steps to be followed to fill the antifreeze agent in the hydronic heating system,
The first step is to close the feed valve, which is located near the boiler
In most hydronic heating systems, the location for forcing the antifreeze is the boiler drain valve. Identify this valve properly
Start pushing the antifreeze in the boiler by using boiler drainage
You should add the required antifreeze so that the system’s pressure reaches around 12 to 15 PSI (Pound per square inches).
hydronic heating control systems | controls in hydronic heating system
Hydronic heating system control should be capable of controlling the efficiency and the comfort inside the home.
We should have proper control of the system, either it managing single room or multi rooms.
hydronic heating system glycol | antifreeze hydronic heating system
In a cold climate, the antifreeze agent is needed so that the water will not get freeze inside the tube and blockage. Generally, glycol is used as antifreeze in the hydronic heating system. The glycol posses a lower freezing temperature, so that system working fluid stays in the liquid phase in extremely cold conditions.
purge air hydronic heating system | how to get air out of hydronic heating system | how to remove air from hydronic heating system
The Purging of air from the system can be done with the following steps,
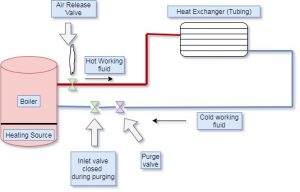
- First of all, finds and identifies the air bleed valve near to radiator. This valve is small and cylindrical with one inch in height. The head of this valve is a slotted screw with a small nozzle inside it.
- After finding the air bleed valve, turn the air bleed valve in an anti-clockwise direction to open it. If air is present in the heating system, the air will come out with the valve opening through the nozzle. The water particles also come out with air. Keep valve open for some time till the complete water starts coming out without air. Close the valve once you notice the steady water flow.
- Closing of the valve can be done by rotating the air valve in a clockwise direction. In some heating systems, there are multiple valves installed in the system.
For related topics, please click here