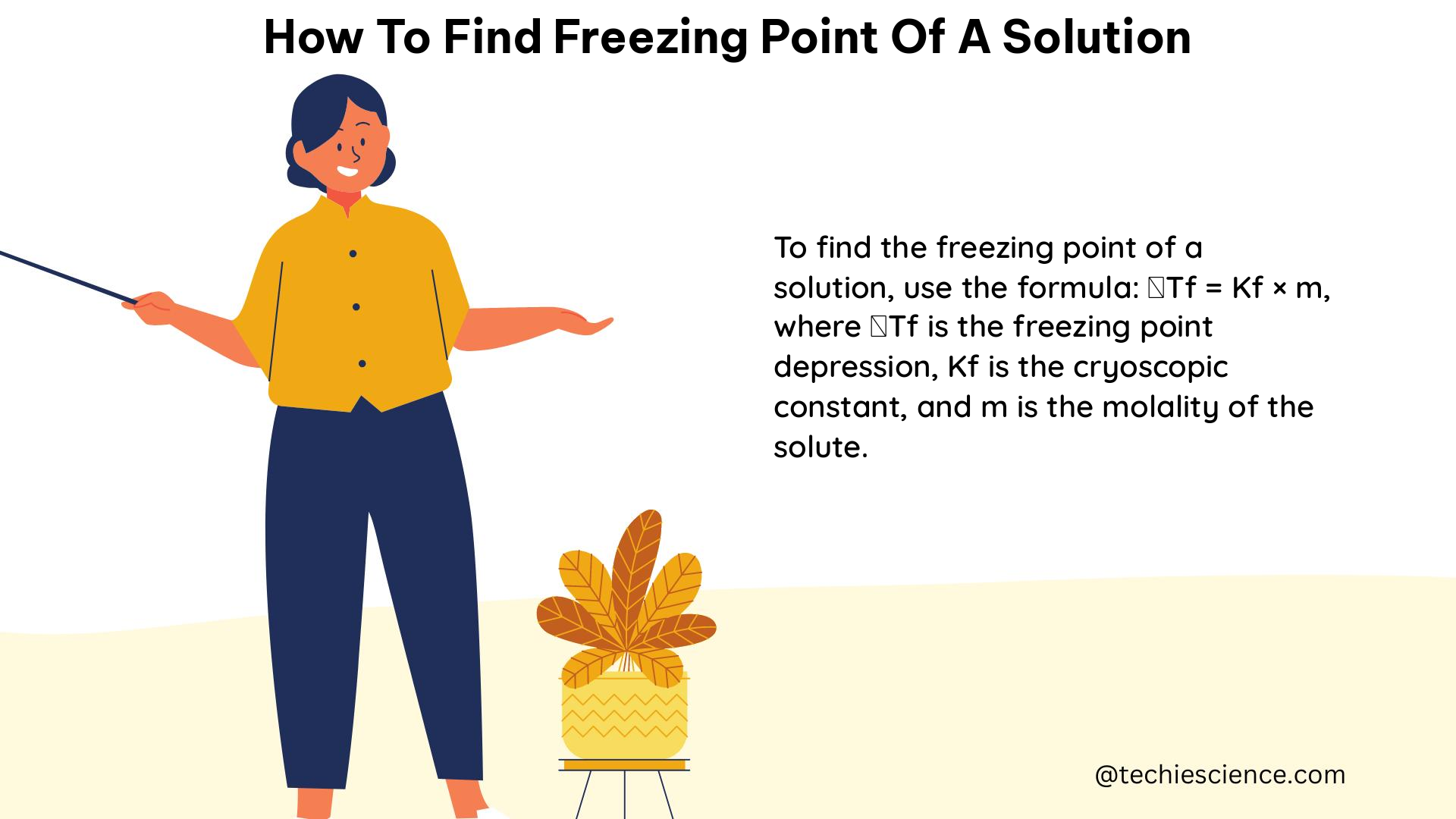
Buoyancy is exemplified by submarines, which adjust buoyancy to dive or surface, floating icebergs displacing water equal to their weight, and hot air balloons rising due to lower density than surrounding air. Archimedes’ principle quantifies buoyancy, stating that the upward buoyant force equals the weight of the fluid displaced by the submerged part of the object.
- Floating a ball on water
- Helium gas balloon
- The egg floating in the water
- Sailing of boat or ship
- Submarines
- Steel object on the water
- Duck toy in the bathtub
- Oil in water
- Floating of rubber
- Aircraft
- Fired bullet in the air
- Fishes
- A swimmer
- Wooden log floating
- Paper piece
- Iceberg floating on water
- Clouds
- Aquatic plants
- Life jackets
- Water decorative items
- Plastic bottles
- Aluminum foils
- Hydrometers
- Leaves
- Sky lanterns
- Underwater divers
- Skydivers
- Saltwater
Floating a ball on water
A ball floats on water because of buoyancy. The density of water is more than the ball, so it floats on the water.

Helium gas balloon
Helium is lighter gas whose density is much smaller than the air, so it sails in the air easily. The sailing is due to the buoyancy of the balloon on air medium.

Egg floating
The egg has a special buoyancy character; it always sinks in fresh water and floats on saltwater; this is due to the differences in the density of water and saltwater.
Sailing of boat or ship
Ships and boats sail on the water due to buoyancy. The buoyancy exerted on the ships and boat makes them sail efficiently.
Submarines
Submarines are underwater vehicle that floats due to buoyancy.

Image credits: Pixabay
Steel object on the water
The steel objects sink into the object because steel is denser than the water. The sink is due to less buoyancy of steel in water.
Duck toy in the bathtub
The duck toys float on the water in the bathtub due to buoyancy.

Image credits: Pixabay
Oil spills
Oil spills float on the water because of the buoyancy exerted on the oil spills.
Floating of rubber
Some rubber objects float on the water because of the buoyancy exerted on them.
Aircraft
The lift and the drag acting on the aircraft while moving in the air are due to the buoyancy.
Fired bullet in the air
When the bullet is fired, it travels in the air. The buoyancy exerted on the bullet makes them move in the air.
Fishes
The fish can swim easily on the water due to buoyancy. The thick swim bladder is expanded and displaces the water, and thus, greater buoyancy is exerted.
A swimmer
A swimmer experiences more buoyancy while swimming. It makes them float on the water easily.
Wooden log floating
Wooden logs are the best buoyancy examples. The float of wooden logs on the water is due to buoyancy.
Paper piece
Paper pieces experience buoyancy at different stages. Initially, it floats on the water; as it absorbs water, it begins to sink. Both are due to buoyancy.
Iceberg floating on water
The icebergs are nothing but the solid form of water. But the density of the solid form is less than the liquid form; hence they float on the water with maximum buoyancy.
Clouds
The floatation of clouds in the sky is due to buoyancy acting on them. The latent releases during the formation of clouds, and the temperature falls, making the air rise. Thus floatation of clouds is additionally enhanced by the buoyancy.
Aquatic plants
Aquatic plants experience a maximum amount of buoyancy which counteracts their weight. The buoyancy developed is responsible for their cells to flexible and soft.
Life jackets
The life jackets provided while swimming and water surfing have the buoyancy to make the user float properly on the water.
Water decorative items
Some of the items used for decoration on the water are of little less dense than the water; thus, they have the buoyancy to float on the water.
Plastic bottles
An empty plastic bottle can float on the water because its average density is less than the water.
Aluminum foils
The buoyancy acting on the aluminum foil depends on its density. A foil with less density than water can float, and the foil with more density sinks in the water.
Hydrometers
A hydrometer is an instrument that is used to measure the density of a liquid. It is one of the best buoyancy examples because it measures the density by the ability of the liquid to achieve buoyancy.

Image credits: Flickr
Leaves
Some of the leaves float on water as well as in the air, and some type of leaves sinks in water after they fall from the tree. The floatation and sink of the leaves depend on the buoyancy.
Sky lanterns
The pressure created when the sky lanterns are ignited, the lanterns rise in the air, and buoyancy helps to keep them sailing in the sky.
Underwater divers
Underwater divers experience the maximum amount of buoyancy to swim in the deep ocean and river.
Skydivers
When a diver dives into the air, he experiences a change in the buoyancy for every second as he is near to achieving the terminal velocity.
Saltwater
Comparing the freshwater with saltwater, saltwater has maximum buoyancy to make the object float. This is because when the salt is added to the water, the density of the water increases.
Neutral buoyancy examples
Neutral buoyancy occurs only when the object’s density is balanced by the medium density, and thus sub immersed situation of the object occurs. In this section, such neutral buoyancy examples are listed.
- Scuba divers
- A fish
- Human body fluid
- Hot air balloons
- Hardwoods
- Submarines
- The egg floating on saltwater
- Bullets
- Plastic containers
- Hollow steel ball
- Watermelon
- Balloons in water
- Rubber bands immersed in water
- Toothpicks with plastic material
- Underwater robots
- Submerged flexible piping
- Lemons
- Human body
- Soap bubbles in the air
- Astronaut’s training
Scuba divers
A scuba diver has neutral buoyancy. When the diver dives into the sea, he is balanced by gravity, which makes him neither sink nor rise.

Image credits: Pixabay
A fish
A fish is a very good way to explain the neutral buoyancy examples as they are provided with a pair of swing bladders which is operated by balancing the air that makes the fish not sink or rise.
Human body fluid
The human can efficiently swim in the water because of his body fluid and tissues as they are neutral buoyancy in nature.
Hot air balloons
As soon as the hot air balloons are rise in the air, they keep on sailing at a constant rate until they balanced by the air density because they are under neutral buoyancy.

Image credits: Pixabay
Hardwoods
Some of the hardwoods are not completely sinking in water; not they float on the water, but they remain in the submerged state, which is nothing but neutral buoyancy.
Submarines
Submarines are excellent neutral buoyancy examples because they are made to travel in the mid-way of the sea, neither sinking nor rising.
The egg floating on saltwater
When you drop the egg into the saltwater, it will remain in sub immersed state because the density of the egg is balanced by the salt water, causing then not to sink.
Bullets
When the bullets are fired in the air, they will hit the target, falling or rising in the air.
Plastic containers
Some of the plastic containers, when they are subjected to floating on water, will be in the submerged condition due to the balance of density between the container and water.
Hollow steel ball
Even though steel is denser than water and sinks in them, they are balanced by the neutral buoyancy when the steel is of hallow because hollow steel allows the air to balance the density of water to achieve neutral buoyancy.
Watermelon
Watermelon consists of a large amount of water content in it, so when they are immersed in the water, their density will be somewhat equal, and hence watermelon is partially neutral buoyancy in water.
Balloons in water
When the balloon is immersed in water with a plastic material tied to it, the density of the balloon together with the plastic is balanced by the water density to enhance neutral buoyancy.
Rubber bands immersed in water
Suppose a rubber band is immersed in water; initially, they sink, but after some time, they are balanced by the air molecule and rise in the middle, causing them to float.
Toothpicks with plastic material
The toothpicks with plastic material made to immerse in the water, both their densities together become equal to water density, and hence they will float in the water with neutral buoyancy.
Underwater robots
The underwater robots are the remote-controlled robots used for research purposes. They are designed to achieve neutral buoyancy when they fall in the water.
Submerged flexible piping
The submerged flexible pipelines are made up of rubber or plastic, which is enhanced by neutral buoyancy, which accomplishes the self-controlling flexible arrangements.
Lemons
When they drop into the saltwater, Lemons neither try to sink nor try to rise, causing neutral buoyancy.
Human body
The human body with floating materials attains neutral buoyancy. A person without any knowledge of swimming can float, neither sinking nor rising in the water with the floating material.
Soap bubbles in the air
Soup bubbles in the air have neutral buoyancy as they are very light, and density is much equal to air density. They cannot rise in the air as they are very short-lived.
Astronaut’s training
The astronauts are trained by creating the microgravity environment, which traces the neutral buoyancy condition by floating above the surface.
Negative buoyancy examples
Any objects that sink in a fluid medium are referred to as negative buoyancy examples. A list of negative buoyancy examples is given in this section.
- A brick in water
- Pebbles
- Spheres dropped in water
- Coins in water
- Diving submarines
- Keys
- Eggs in freshwater
- Lemmon in freshwater
- Paper clips
- Sand
- Bones and muscles
- Shot put
- Steel tanks
- Iron screws
- Rice grains in water
- Plastic bottles filled with stones
- Fishing nets
- Anchors sink on the ocean floor
A brick in water
A brick can completely sink in the water because its density is much heavier than the water, thus creating negative buoyancy.
Pebbles
When the pebbles are dropped in water, they sink immediately, having a density more than the water.
Spheres dropped in water.
Spheres of iron or steel are much heavier, and they cannot sustain to float on the water, causing them to sink.
Coins in water
The coins sink as soon as you drop them into the water as their density is heavier than the water, so coins in water are negative buoyancy examples.
Diving submarines
When the submarines are about to immerse in the water, they are negatively buoyant. This is because if they do not have negative buoyancy, it is impossible for them to move under the water.
Keys
The keys are negatively buoyant because they sink in the water when you drop them in the water.
Eggs in freshwater
Eggs do not float on the freshwater; they immediately sink as soon as they drop inside the water.
Lemmon in freshwater
Same as eggs, lemons also sink in the freshwater because the density of the lemon is much greater than the water.
Paper clips
Clips used to hold the paper on the board are negative buoyancy as they sink in the water.
Sand
The sand particles are negative buoyancy as they do not Float in the water but sink. So it is the reason behind the sand settling under the water resources.
Bones and muscles
Bones are muscles of a person are negatively buoyancy examples because they are much denser and they are the reason for a man to sink if he does not know swimming.
Shot put
A shot put is much heavier and also denser than the water and air. So they do not fly or float.
Steel tanks
Steel tanks sink in water because of their density. So they have negative buoyancy in water.
Iron screws
Iron screws appear to be light, but their density is more than the water, so they sink and have negative buoyancy in water.
Rice grains in water
If you observe the rice grain, you will get to know it has negative buoyancy. Because when you put rice grains in water, they sink.
Plastic bottles filled with stones
Plastics have very good buoyancy to float on the water, but if you fill the plastic bottle with some stones, it immediately sinks because of stones.
Fishing nets
To catch the fish from the sea or river, one has to throw the net. These nets are negative buoyancy and sink immediately as soon as they throw in the water.
Anchors sink on the ocean floor.
The anchors of the ships and boats are negatively buoyancy as they sink in the ocean. They are one of the reasons for the safe sailing of ships and boats.
Image credits: Flickr
Buoyant force examples
The buoyant force is an upward force exerted on the object following the famous Archimedes principle. This section provides a detailed explanation of buoyant force examples.
- Submarines
- Sailing of parachutes
- Clouds
- Balloons
- Hairpins
- Iron ball
- Tennis ball
- Cork
- Sawdust
- Rubber bands
- Firearm bullets
- Rocks near the ocean
- Froth on water
- Floating wood
- Person in water
- Plastic box
- Sheet of paper
- Air parcel
- Sea divers
- Aircraft and rockets
Submarines
The submarines are able to sail under the sea because of the buoyant force. The buoyant force is balanced by the upward thrust and is able to maintain the floatation of the submarines.
Sailing of parachutes
The upward thrust, lift, and drag are balanced by the buoyant force of the parachutes and help to sail in the air.
Clouds
The floatation and generation of cloud are due to the buoyant force applied to the clouds carried by the wind.

Balloons
The rise and fall of the balloon in the air depends on the buoyant force acting on them.
Hairpins
When the hairpins are dropped in the water, they sink. The buoyant force between the hairpin and water is less and causes sinking. Sinking is also due to the buoyant force.
Iron ball
The heavier iron ball sinks into the water. The ball has neutral buoyancy, and hence buoyant force acting between them cannot balance the upward thrust, so they sink.
Tennis ball
The tennis ball is good at floating on the water. The upward thrust and gravity are equally balanced by the buoyant force; thus, they can float.
Cork
The density of the cork is less than the water, so the upward buoyant force makes the cork float on the water.
Sawdust
Saw specks of dust are lighter, and the density is less compared to water. So when they are put on the water, they float because the upward buoyant force acting on the sawdust is more.
Rubber bands
Rubber bands can float over water because of the buoyant force. Because of the density of rubber bands, the upwards thrust will be more and makes them float.
Firearm bullets
Bullets out from the firearm are well balanced by the buoyant force. So they keep on moving in the air medium until they are hit by the target.
Rocks near the ocean
The rocks near the large ocean are a good example of the buoyant force as they do not sink in the water. The buoyant force acting in the upward direction holds the rocks on the surface.
Froth on water
The froth created on the water is assumed to be massless and can float on the water for a while. The upward force on the water pushes the froth to float on the water.

Image credits: Flickr
Floating wood
When the wood is floating on the water, the buoyant force opposes the wood from sinking and remaining in the partially immersed state.
Person in water
The weight of the person is necessary to exert an upward force to oppose the person from sinking in the water. The buoyant force always acts opposite to the weight of the object. Thus buoyant force makes the person float.
Plastic box
The plastic box of less density experiences the buoyant force in the fluid medium in the opposite direction and makes them float.
Sheet of paper
The sheet of paper can experience a buoyant force when they fall on the water. They float on water if they experience maximum buoyant force.
Air parcel
The air parcels are of the same density as the surrounding air molecule; hence they experience the buoyant force. This buoyant force is neutral, and it does not even rise nor sink.
Sea divers
The sea divers have much knowledge of buoyant force as they experience a maximum buoyant force when they dive. If they go underwater, the buoyant force will be neutral.
Aircraft and rockets
The aircraft and the rockets in the air experience the buoyant force. An aircraft or rocket is balanced by the four forces in the air for their efficient motion; one of them is the buoyant force which keeps them constantly moving in the air without falling.
Also Read:
- How to fix uneven pad wear in hydraulic disc brakes
- How do polarized sunglasses reduce glare compared to regular sunglasses
- Stable equilibrium example
- Telescopic sights
- Harmonic oscillator examples
- Microscope in physics
- Conduction
- How to find coefficient of friction
- When does a physical change occur
- Clouds and fog