Sulfur is a chemical element with the symbol S and atomic number 16. It belongs to the group 16 elements in the periodic table, also known as the chalcogens. The electron configuration of sulfur is 1s^2 2s^2 2p^6 3s^2 3p^4. This means that sulfur has two electrons in its 1s orbital, two in its 2s orbital, six in its 2p orbital, two in its 3s orbital, and four in its 3p orbital. The electron configuration of sulfur gives it a total of 16 electrons.
Key Takeaways

| Orbital | Number of Electrons |
|---|---|
| 1s | 2 |
| 2s | 2 |
| 2p | 6 |
| 3s | 2 |
| 3p | 4 |
Understanding Electron Configuration
Electron configuration is a fundamental concept in chemistry that describes the arrangement of electrons within an atom. It provides valuable information about the distribution of electrons in different energy levels and orbitals. By understanding electron configuration, we can gain insights into the chemical behavior and properties of elements.
Explanation of Electronic Configuration Notation

Electronic configuration notation is a way to represent the arrangement of electrons in an atom. It uses a combination of numbers, letters, and superscripts to denote the specific electron distribution. The notation is based on the principles of the periodic table, atomic number, and electron shell structure.
In this notation, the atomic number represents the number of protons in the nucleus of an atom. Each atomic number corresponds to a specific element on the periodic table. For example, sulfur, with an atomic number of 16, has 16 protons in its nucleus.
The electron configuration notation also takes into account the concept of electron shells. Electron shells are energy levels that surround the nucleus of an atom. The first shell, closest to the nucleus, can hold a maximum of 2 electrons, while the second shell can hold up to 8 electrons. Subsequent shells have higher energy levels and can accommodate more electrons.
To represent the electron arrangement, the notation uses a series of numbers and letters. The numbers indicate the electron shell, while the letters denote the type of subshell or orbital. For instance, the electron configuration of a sulfur atom is 1s² 2s² 2p⁶ 3s² 3p⁴. This notation tells us that sulfur has 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, 6 electrons in the 2p orbital, 2 electrons in the 3s orbital, and 4 electrons in the 3p orbital.
Interpretation of Notation
To interpret the electron configuration notation, we need to understand the quantum numbers and atomic orbitals. Quantum numbers describe the energy, shape, and orientation of an electron within an atom. They help determine the specific arrangement of electrons in different orbitals.
Atomic orbitals are regions within an electron shell where electrons are most likely to be found. Each orbital can hold a maximum of 2 electrons with opposite spins. The four types of atomic orbitals are s, p, d, and f, each with a different shape and orientation.
By analyzing the electron configuration, we can determine important properties of an element. For example, the number of valence electrons, which are electrons in the outermost shell, determines an element‘s chemical reactivity. In the case of sulfur, it has 6 valence electrons in the 3p orbital, making it highly reactive and capable of forming various compounds.
Understanding electron configuration is crucial for comprehending the atomic structure and chemical behavior of elements. It provides a basis for explaining the properties and reactions of elements, as well as predicting their involvement in chemical reactions and the formation of compounds.
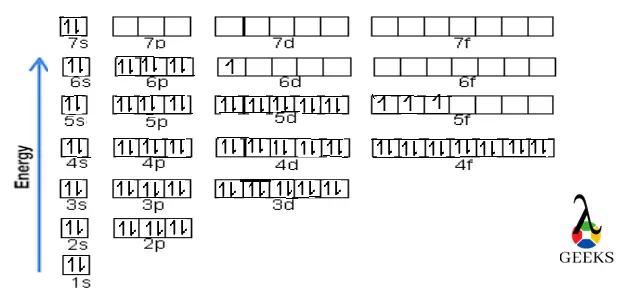
To visualize electron configuration, an electron configuration chart can be used. This chart organizes the electron arrangement for each element, allowing for easy reference and comparison. It provides a comprehensive overview of the electron distribution in different elements and helps in understanding the patterns and trends in the periodic table.
Sulfur Electron Configuration
Sulfur, with an atomic number of 16, has an electron configuration that determines its chemical properties and behavior. The electron configuration of an atom describes how its electrons are distributed among the various energy levels and orbitals within the atom.
Sulfur Electron Configuration Notation
The electron configuration notation for sulfur is [Ne] 3s^2 3p^4. This notation represents the electron arrangement of sulfur by indicating the noble gas that precedes it in the periodic table (Neon in this case), followed by the electron configuration of the remaining orbitals.
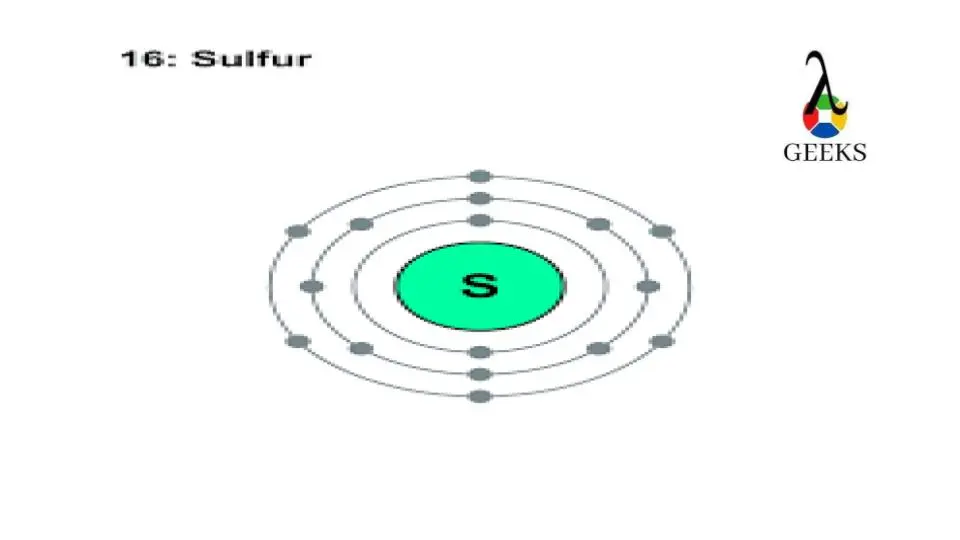
Sulfur Electron Configuration Diagram
To visualize the electron configuration of sulfur, we can use a diagram that represents the different energy levels and orbitals. In the case of sulfur, the 3s orbital is filled with 2 electrons, and the 3p orbital is filled with 4 electrons.
| Energy Level | Subshell | Number of Electrons |
|---|---|---|
| 1 | 1s | 2 |
| 2 | 2s | 2 |
| 2 | 2p | 6 |
| 3 | 3s | 2 |
| 3 | 3p | 4 |
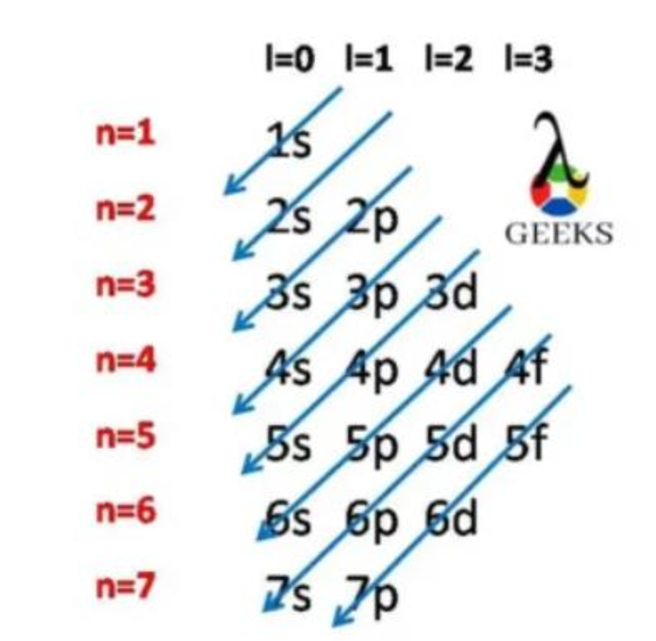
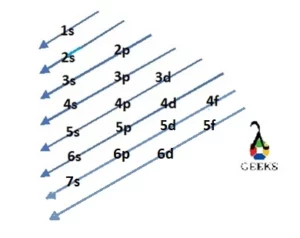
Step-by-Step Explanation of Filling Orbitals
The electron configuration of sulfur can be understood by following a step-by-step process of filling the orbitals according to the rules of quantum mechanics.
- The first two electrons of sulfur occupy the 1s orbital, which is the lowest energy level.
- The next two electrons fill the 2s orbital, which is the next available energy level.
- The remaining six electrons are distributed among the three 2p orbitals, with each orbital accommodating a maximum of two electrons.
- Finally, the last two electrons occupy the 3s orbital.
Presentation of Sulfur Orbital Diagram
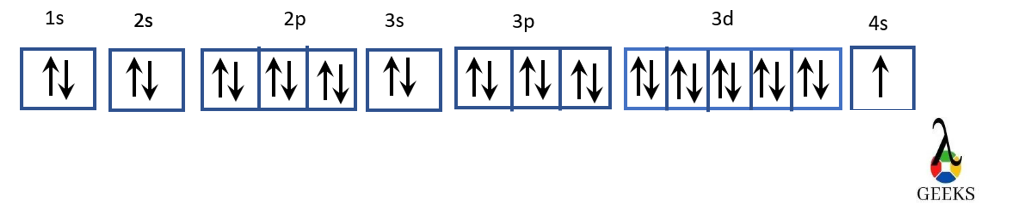
The electron configuration of sulfur can also be represented using an orbital diagram. In this diagram, each orbital is represented by a box, and the electrons are represented by arrows. The direction of the arrow indicates the electron spin.
1s: ↑↓
2s: ↑↓
2p: ↑↓ ↑↓ ↑
3s: ↑↓
3p: ↑↓ ↑↓ ↑↓
The orbital diagram shows that the 1s and 2s orbitals are filled with two electrons each, while the 2p orbital is filled with six electrons. The remaining two electrons occupy the 3s orbital.
Understanding the electron configuration of sulfur is essential for comprehending its atomic structure and the chemical properties it exhibits. The arrangement of electrons in an atom determines how it interacts with other atoms and forms compounds. Sulfur, with its electron configuration, plays a crucial role in various sulfur compounds and their chemical behavior.
Remember, the electron configuration of sulfur can be represented using the notation [Ne] 3s^2 3p^4, and it can also be visualized through an electron configuration diagram and an orbital diagram. These representations provide valuable insights into the electron arrangement and distribution within a sulfur atom.
Sulfur as an Ion
Explanation of Ion Formation
Sulfur, with an atomic number of 16, is a nonmetal element found in the periodic table. When sulfur atoms undergo ionization, they can either gain or lose electrons to form ions. The ionization process occurs when the outermost electron shell of a sulfur atom is either filled or emptied, resulting in a stable electron configuration.
The electron configuration of a sulfur atom is 1s^2 2s^2 2p^6 3s^2 3p^4. This means that sulfur has a total of 16 electrons distributed among its various electron orbitals. The chemical properties of sulfur are determined by the arrangement of these electrons in its atomic structure.
During ion formation, sulfur atoms can either gain two electrons to achieve a stable electron configuration similar to that of a noble gas, or lose six electrons to expose a full outermost shell. This process allows sulfur to attain a more stable electron arrangement, which in turn affects its chemical behavior.
Effect of Ionization on Electron Configuration
When sulfur gains two electrons, it forms a negatively charged ion known as sulfide (S^2-). This ion has an electron configuration of 1s^2 2s^2 2p^6 3s^2 3p^6, which is the same as the electron configuration of the noble gas argon (Ar). By gaining two electrons, sulfur fills its outermost electron shell, achieving a more stable configuration.
On the other hand, when sulfur loses six electrons, it forms a positively charged ion called sulfite (S^6+). The electron configuration of sulfite is 1s^2 2s^2 2p^6, which is the same as the electron configuration of the noble gas neon (Ne). By losing six electrons, sulfur exposes a full outermost shell, resulting in a more stable electron arrangement.
Charge of Sulfur Ion
The charge of a sulfur ion depends on whether it gains or loses electrons during ionization. When sulfur gains two electrons, it becomes a sulfide ion with a charge of -2. Conversely, when sulfur loses six electrons, it becomes a sulfite ion with a charge of +6. The charge of the sulfur ion is determined by the number of electrons gained or lost in order to achieve a stable electron configuration.
To summarize, the ionization of sulfur atoms leads to the formation of sulfide and sulfite ions, which have different electron configurations and charges. These ions play a significant role in the chemical behavior of sulfur and its ability to form various sulfur compounds. By understanding the electron arrangement and charge of sulfur ions, we can gain insights into the unique properties and reactivity of this element.
Sulfur Electron Configuration in Different States
Sulfur Electron Configuration in Sulfur Dioxide
When it comes to understanding the electron configuration of sulfur in different states, let’s start with sulfur dioxide (SO2). Sulfur dioxide is a compound composed of one sulfur atom and two oxygen atoms. To determine the electron configuration of sulfur in sulfur dioxide, we need to consider the atomic structure of sulfur and its interaction with oxygen.
Sulfur, with an atomic number of 16, has an electron configuration of 1s2 2s2 2p6 3s2 3p4. This means that sulfur has two electrons in its 1s orbital, two electrons in its 2s orbital, six electrons in its 2p orbital, two electrons in its 3s orbital, and four electrons in its 3p orbital.
In sulfur dioxide, the sulfur atom forms covalent bonds with two oxygen atoms. Each oxygen atom contributes two electrons to form a double bond with sulfur. As a result, the electron configuration of sulfur in sulfur dioxide can be represented as 1s2 2s2 2p6 3s2 3p2. The two electrons in the 3p orbital are involved in the bonding with oxygen, leaving the other two p-orbital electrons unpaired.
Sulfur Electron Configuration in Excited State
Now, let’s explore the electron configuration of sulfur in an excited state. When an atom is in an excited state, it means that one or more of its electrons have absorbed energy and moved to a higher energy level or orbital. In the case of sulfur, the excited state can be achieved by promoting an electron from the 3p orbital to the 3d orbital.
The electron configuration of sulfur in the excited state can be represented as 1s2 2s2 2p6 3s2 3p3 3d1. Here, one electron from the 3p orbital has moved to the 3d orbital, resulting in an unpaired electron in the 3d orbital. This excited state configuration of sulfur can have implications for its chemical properties and reactivity.
Sulfur Electron Configuration in Oxygen
Lastly, let’s discuss the electron configuration of sulfur in the presence of oxygen. Oxygen, with an atomic number of 8, has an electron configuration of 1s2 2s2 2p4. When sulfur interacts with oxygen to form compounds like sulfur oxides, the electron configuration of sulfur is influenced by the electron arrangement of oxygen.
In the case of sulfur oxide compounds, such as sulfur trioxide (SO3) or sulfur hexafluoride (SF6), the sulfur atom forms bonds with oxygen atoms. The electron configuration of sulfur in these compounds can vary depending on the specific compound and bonding arrangement. However, the overall electron configuration of sulfur will still follow the basic pattern of 1s2 2s2 2p6 3s2 3p4, with additional electrons from the oxygen atoms.
Detailed Look at Sulfur Electron Configuration
Sulfur, with an atomic number of 16, has an interesting electron configuration that determines its chemical properties and behavior. Let’s take a closer look at the electron configuration of sulfur and explore its various forms and representations.
Sulfur Electron Configuration Long Form
In the long form, the electron configuration of sulfur is written as 1s2 2s2 2p6 3s2 3p4. This notation represents the distribution of electrons in different electron shells and orbitals within the sulfur atom. The numbers and letters indicate the principal quantum number (n) and the subshell (s or p) in which the electrons are located.
Sulfur Electron Configuration Short Form
The short form of sulfur’s electron configuration is [Ne] 3s2 3p4. Here, the noble gas notation [Ne] represents the electron configuration of the previous noble gas, neon. It simplifies the notation by indicating that the core electrons (those in the inner shells) remain the same as neon, while only the valence electrons (those in the outermost shell) are added.
Sulfur Electron Configuration Full
The full electron configuration of sulfur can be represented as 1s2 2s2 2p6 3s2 3p4. This notation provides a detailed breakdown of the number of electrons in each energy level and sublevel of the atom. It helps us understand the arrangement of electrons and their distribution across different orbitals.
Sulfur Electron Configuration with Arrows
To visualize the electron configuration of sulfur, we can use arrows to represent the electrons in each orbital. In the case of sulfur, we would have two arrows in the 1s orbital, two arrows in the 2s orbital, six arrows in the 2p orbital, two arrows in the 3s orbital, and four arrows in the 3p orbital. This representation helps us visualize the filling of electron orbitals according to the Aufbau principle.
Sulfur Electron Configuration Periodic Table
On the periodic table, sulfur is located in the third period and the sixteenth group. Its electron configuration can be determined by its position in the periodic table, following a pattern based on the periodic table’s structure. By understanding the periodic table and the arrangement of elements, we can easily determine the electron configuration of sulfur and other elements.
Understanding the electron configuration of sulfur is crucial in comprehending its atomic structure and chemical behavior. The arrangement of electrons in different orbitals and energy levels influences the element’s reactivity, bonding capabilities, and overall chemical properties. Sulfur’s electron configuration, with its specific arrangement of electrons, contributes to its ability to form various compounds and participate in chemical reactions.
Frequently Asked Questions
What is the electron configuration of sulfur atom in ground state?
The electron configuration of an atom describes how its electrons are distributed among the various energy levels and sublevels within the atom. In the case of sulfur, which has an atomic number of 16, the electron configuration in its ground state is 1s^2 2s^2 2p^6 3s^2 3p^4. This means that sulfur has two electrons in the 1s orbital, two electrons in the 2s orbital, six electrons in the 2p orbital, two electrons in the 3s orbital, and four electrons in the 3p orbital.
When sulfur becomes an ion, what is its charge?
When sulfur becomes an ion, it tends to gain two electrons to achieve a stable electron configuration. By gaining two electrons, sulfur achieves a full outer electron shell, similar to the noble gas argon. As a result, sulfur becomes negatively charged and forms an ion with a charge of -2.
What is the notation for sulfur’s electron configuration?
The notation used to represent sulfur’s electron configuration is based on the periodic table and the arrangement of electrons in different energy levels and sublevels. The electron configuration of sulfur can be written as 1s^2 2s^2 2p^6 3s^2 3p^4, where the numbers and letters represent the energy levels and sublevels, and the superscripts indicate the number of electrons in each orbital.
What is the complete electron configuration of sulfur?
The complete electron configuration of sulfur is 1s^2 2s^2 2p^6 3s^2 3p^4. This configuration represents the distribution of all 16 electrons in the atom, with two electrons in the 1s orbital, two electrons in the 2s orbital, six electrons in the 2p orbital, two electrons in the 3s orbital, and four electrons in the 3p orbital.
When sulfur forms an ion, what is its charge?
When sulfur forms an ion, it gains two electrons to achieve a stable electron configuration. By gaining these two electrons, sulfur achieves a full outer electron shell and becomes negatively charged. Therefore, the charge of a sulfur ion is -2.
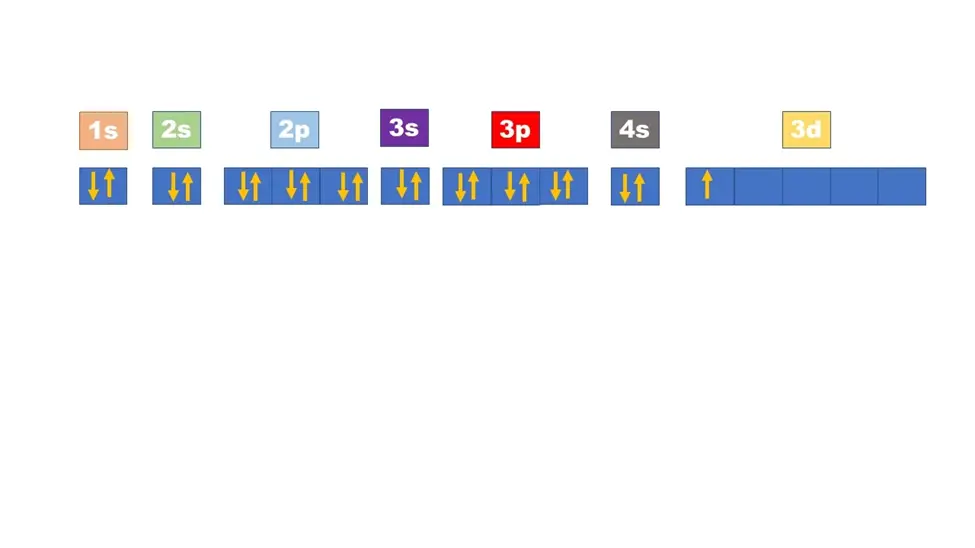
How can I represent sulfur’s electron configuration using arrows?
Sulfur’s electron configuration can be represented using arrows in an electron configuration chart. The chart consists of energy levels and sublevels, with arrows indicating the direction of electron spin. For sulfur, the electron configuration can be represented as:
1s ↑↓
2s ↑↓
2p ↑↓ ↑↓ ↑↓
3s ↑↓
3p ↑↓ ↑↓ ↑↓
The arrows represent the electrons, with the up arrow (↑) indicating one spin direction and the down arrow (↓) indicating the opposite spin direction.
What is the electron configuration of sulfur in Class 10?
In Class 10, the electron configuration of sulfur is usually taught as 2, 8, 6. This shorthand notation represents the number of electrons in each energy level, starting from the innermost level. In this notation, the first energy level (1s) has 2 electrons, the second energy level (2s and 2p) has 8 electrons, and the third energy level (3s and 3p) has 6 electrons.
Can you provide a diagram of sulfur’s electron configuration?
Certainly! Here is a diagram representing the electron configuration of sulfur:
1s^2
2s^2 2p^6
3s^2 3p^4
In this diagram, each energy level is represented by a row, and the orbitals within each energy level are shown. The superscripts indicate the number of electrons in each orbital.
When sulfur gains an electron from beryllium, what does it become?
When sulfur gains an electron from beryllium, it becomes a sulfur ion. In its neutral state, sulfur has 16 electrons, but when it gains an electron, it now has 17 electrons. This change in electron count affects the overall charge of the sulfur atom, making it negatively charged.
When sulfur becomes an ion, will electrons be lost or gained?
When sulfur becomes an ion, it gains an electron. This process occurs when sulfur, with its atomic number of 16, accepts an electron from beryllium, which has an atomic number of 4. By gaining an electron, sulfur achieves a stable electron configuration, similar to the noble gas argon. This stability is achieved by filling the electron orbitals in the outermost energy level, known as the valence shell.
To understand the electron arrangement of sulfur, we can refer to the periodic table. Sulfur belongs to Group 16, also known as the chalcogens. It has a total of three electron shells, with the first shell containing two electrons, the second shell containing eight electrons, and the third shell containing six electrons. By gaining one more electron, sulfur completes its third shell with eight electrons, satisfying the octet rule.
The electron configuration chart for sulfur can be represented as follows:
| Shell | Subshell | Number of Electrons |
|---|---|---|
| 1 | 1s | 2 |
| 2 | 2s | 2 |
| 2 | 2p | 6 |
| 3 | 3s | 2 |
| 3 | 3p | 5 |
The valence electrons of sulfur are the electrons in the outermost energy level, which in this case is the third shell. By gaining one electron, sulfur now has three valence electrons, allowing it to form stable compounds with other elements.
In terms of atomic structure, sulfur gains an electron by filling the 3p subshell, specifically the 3p^5 subshell. The quantum numbers associated with these electrons determine their position and energy within the atom. The electron cloud surrounding the sulfur ion reflects the distribution of these electrons in various atomic orbitals.
The atomic model and electron arrangement of sulfur are essential in understanding the chemical behavior of this element. By gaining an electron, sulfur becomes more stable and exhibits different chemical properties compared to its neutral state. The additional electron influences the electron spin and subshell configuration, leading to changes in the overall reactivity and bonding capabilities of sulfur.
Overall, when sulfur gains an electron from beryllium, it becomes a sulfur ion with a negative charge. This transformation is driven by the desire to achieve a more stable electron configuration, following the principles of atomic theory and the periodic table. The gained electron fills the valence shell, allowing sulfur to participate in various chemical reactions and form compounds with other elements.
Frequently Asked Questions
What is the sulfur electron configuration long form?
The long form electron configuration of sulfur (S), which has an atomic number of 16, is 1s² 2s² 2p⁶ 3s² 3p⁴. This configuration shows the distribution of electrons in the atomic orbitals of sulfur.
How does the sulfur electron configuration change in an excited state?
In an excited state, an electron from a lower energy level in sulfur is excited to a higher energy level. For example, one electron from the 3s² or 3p⁴ could be excited to a 4s or 4p orbital, altering the electron configuration.
Does sulfur have a specific electron configuration?
Yes, sulfur has a specific electron configuration. The ground state electron configuration for a sulfur atom is 1s² 2s² 2p⁶ 3s² 3p⁴.
What does the sulfur electron configuration with arrows represent?
The sulfur electron configuration with arrows represents the electron spin in each orbital. Each arrow in an orbital box represents an electron, and the direction of the arrow (up or down) indicates the electron’s spin.
What is the sulfur electron configuration notation?
The electron configuration notation for sulfur is [Ne] 3s² 3p⁴. This notation shows that the electron configuration of sulfur is equivalent to the electron configuration of Neon (Ne) with an additional six electrons in the 3s and 3p orbitals.
What is the electron configuration of a sulfur atom in the ground state?
The electron configuration of a sulfur atom in the ground state is 1s² 2s² 2p⁶ 3s² 3p⁴. This configuration represents the most stable state of a sulfur atom.
Where is sulfur located on the periodic table?
Sulfur is located in Group 16 (also known as the Oxygen group) and Period 3 of the Periodic Table.
When sulfur becomes an ion, will electrons be lost or gained?
When sulfur becomes an ion, it typically gains two electrons to achieve a stable electron configuration, becoming a sulfide ion with a charge of -2.
What happens when a sulfur atom reacts with other atoms?
When a sulfur atom reacts with other atoms, it tends to gain two electrons to fill its outer electron shell and achieve a stable electron configuration. This results in the formation of a sulfide ion (S²⁻).
What is the full electron configuration for sulfur?
The full electron configuration for sulfur is 1s² 2s² 2p⁶ 3s² 3p⁴. This configuration shows the distribution of all 16 electrons of a sulfur atom across different atomic orbitals.
Also Read:
- Francium electron configuration
- Seaborgium electron configuration
- Cobalt electron configuration
- Tin electron configuration
- Ni2 electron configuration
- Oxygen electron configuration
- Cerium electron configuration
- Hassium electron configuration
- Arsenic electron configuration
- Molybdenum electron configuration