The most simplest way of expressing a chemical reaction is by using chemical symbols. Let us discuss about the reaction of HBr and HgO.
Hydrogen bromide is a colorless gas with molar mass 80.91 g/mol while mercuric oxide is an orange colored substance with 216.59g/mol molar mass. HgO is insoluble in ether, acetone and ethanol. HBr has a linear molecular shape. The crystal structure of HgO can be orthorhombic, hexagonal and tetragonal.
HgO is mostly used a cathode in Hg batteries and also to produce mercury. Let us discuss about HBr + HgO with all details.
What is the product of HBr and HgO
HBr reacts with HgO to give the products mercuric bromide and water.
HBr + HgO —–> HgBr2 + H2O
What type of reaction is HBr + HgO
HBr + HgO is a double displacement reaction or metathesis.
How to balance HBr + HgO
Balancing a chemical reaction is vital for all reactions. Following is the balancing step of HBr + HgO
- Assigning certain coefficients to both reactants and products
- a HBr + b HgO —-> c HgBr2 + d H2O
- A linear equation is made with the above reaction
- H= a= 2d, Br =a =2c, Hg =b = c, O = b=d
- a =2d, a=2c, b=c, b=d
- Solve the above equation using gauss elimination method
- a=2, b= 1, c= 1, d= 1
- The balanced equation of HBr + HgO is
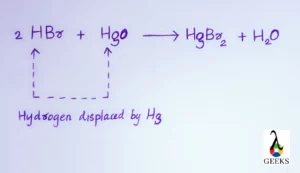
- 2 HBr + HgO —-> HgBr2 + H2O
HBr + HgO titration
HBr + HgO titration cannot be done. HgO is usually treated with iodine first to form HgI2-4 . Then it is titrated with a dibasic acid to give the correct end point. Here HBr is not a dibasic acid. So they cannot titrated each other to get the results.
HBr + HgO net ionic equation
The net ionic equation of HBr and HgO
2H+ + HgO —–> Hg2 ++ H2O.
- The ionic dissociation of HBr and HgBr2 is
- 2H+ + Br– ——> HBr
- Hg2 + + 2Br– ——-> HgBr2
HBr + HgO conjugate Pairs
HBr + HgO reaction has the following conjugate pairs,
- The conjugate base of HBr is Br– or bromide ion.
- The Conjugate acid of HBr is H2Br+or bromonium.
- The conjugate acid of HgO is Hg– .
HBr and HgO intermolecular forces
The intermolecular forces exist in between HBr + HgO is,
- In HBr a dipole is created in between hydrogen and electronegative bromine atom.
- In HgO both metallic bonds and dispersion forces existed.
HBr + HgO reaction enthalpy
The reaction enthalpy of HBr + HgO is -292.79KJ/mol. The enthalpy of formation of HBr, HgO, HgBr2 and H2O is -35.66, -90, -169.45 and -249 kJ/mol respectively. Hence the enthalpy is (-169.45+-249 –(-35.66 + – 90)= -292.79 KJ/mol.
Is HBr + HgO a buffer solution
HBr + HgO is not a buffer solution as HBr a strong acid and HgO is an inorganic substance.
Is HBr + HgO a complete reaction
HBr + HgO is a complete reaction with stable products. All the reactant molecules are converted into corresponding products.
Is HBr + HgO an exothermic or endothermic reaction
HBr + HgO reaction is an exothermic reaction. Since the enthalpy of this reaction calculated is negative it is an exothermic reaction.
Is HBr + HgO a redox reaction
HBr + HgO is not a redox reaction as in this reaction the oxidation state of both the reactants and products are same.
Is HBr + HgO a precipitation reaction
HBr + HgO reaction forms a slightly insoluble precipitate. It is because mercuric bromide formed along with water is slightly soluble in water so it will form a precipitate. Also, mercuric bromide is more denser than water will settle down.
Is HBr + HgO reversible or irreversible reaction
HBr + HgO is an irreversible reaction. This reaction can’t be reversed back to give the reactants.
Is HBr + HgO a displacement reaction
HBr + HgO is a double Displacement reaction. In this reaction the anions and cations of both the reactants are exchanged each other to get the stable products like water and mercuric bromide.
Conclusion
Mercuric oxide is an odourless substance which is solid at room temperature with a Density of about 11.14g/cm3 . Mercuric oxide easily undergo decomposition to give mercury. Oxygen is also produced during this process.
Read more facts on HBr:

Hi… I am Aparna Dev, a chemistry Postgraduate with a good understanding of chemistry concepts. I am working in Kerala Minerals and Metals Limited Kollam with experience in the development of electrocatalysts as a part of post graduate thesis.
Let’s connect through LinkedIn-https://www.linkedin.com/in/aparna-dev-76a8751b9