CH2F2 Lewis structure is effective in emerging the idea about Difluromethane. This article is going to describe 13 reliable facts about the compound that would create impact about the chemical properties of the compound. Those 13 facts are being listed below:
- Drawing CH2H2 Lewis structure
- CH2F2 Lewis structure resonance
- CH2F2 Lewis structure shape
- CH2F2 Lewis structure formal charge
- CH2F2 Lewis structure angle
- CH2F2 Lewis structure octet rule
- CH2F2 valence electrons
- CH2F2 hybridization
- CH2F2 solubility
- CH2F2 ionic or covalent
- CH2F2 acidic or basic
- CH2F2 polar or nonpolar
- CH2F2 tetrahedral
- CH2F2 linear
Drawing CH2H2 Lewis structure
The steps of drawing Lewis structure are quite easy. The fundamental steps can help to identify the Lewis structure of the attainable compound that is Difluromethane in this article.
CH2F2 Lewis structure drawing can be initiated by calculating the number valence electron present in the compound. The number of valance electron in CH2F2 is 20. This fact would be discussed later in this article.
After identifying the total number of valence electrons, it is important to recognise the centre atom by analysing the electronegativity of the atoms. In CH2F2, Carbon has been identified as the centre atom in the compound.
The last step to complete the sketch of the compound would be putting the bond pairs and lone pairs in the drawing. It would complete the drawing of the Lewis structure by re[resenting the electrons by dots.
CH2F2 Lewis structure resonance
Resonance of a compound depends on the presence of pi bond with sigma bond. That bond reliably indicates the transformation of the bond into one electron by keeping the formulas of the compound same as parent compound.
Difluromethane does not have any pi bond. the centre atom Carbon holds the other atoms with four sigma bonds. Therefore, there is no chance to possess any resonating structure of this particular compound.
CH2F2 Lewis structure shape
The shape of compounds are predicted by the Lewis dot structures. This electronic structure can evaluate the overlapping of the orbitals to predict the hybridisation and it is reliable to shape out the compound.
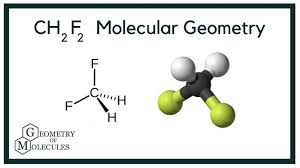
The shape of Difluromethane has been identified as Tetrahedral. This tetrahedral geometry is predicted after completing the structuration of the compound completely.
CH2F2 Lewis structure formal charge
It is very easy to calculate the formal charges of the individual elements in any compound by dividing the total number of bond pairs with 2. The formal charges of the elements indicate the overall charge of the compound which identifies if the compound is positive, negative or neutral.
The formal charge Carbon is CH2F2 is 8/2 = 4 as the number of bond pairs in carbon is 8. Besides, the formal charge of Hydrogen is 2/2 = 1 and it is same for fluorine as both Fluorine and Hydrogen hold 2 bond pairs.
CH2F2 Lewis structure angle
The angle between the bonds of the Lewis structure can be obtained after completing the drawing electronic structure. The electronic structure can bring forth the idea of geometric shape of the compounds.
VSEPR theory is reliable in imposing the effect of lone pairs and bond pairs in verifying the actual bond angle of the compounds. The bond angle of CH2F2 has been identified as109.5 °. This bond angle is effective in sharing the fact that the compound has tetrahedral geometry.
CH2F2 Lewis structure octet rule
Octet rule is the rule if having stable electronic configuration to meet the electron deficiency or excess electron in the compound. this rule says that every element in the periodic table wants to achieve the same electronic configuration like their nearest Noble gas to get the ultimate stability like that element.
Octet rule is considered as the driving force for the elements to undergo electron sharing or transferring process according to their electronic configuration.
In the case of CH2F2, all the elements that are carbon, Hydrogen and Fluorine want to achieve the electronic configuration like helium and Xenon respectively. To fulfil the last energy level with exact number of electrons is the main perspective shared by the elements here to maintain octet rule. This rule works by denoting the number of valence electron in the elements.
Read more about Facts On C4H6O4 + NaOH
CH2F2 valence electrons
The number of valence electrons in th elements is the fact which is the reason for making them reliable to identify their deficiency of excesses of electrons in their last electron shell. The number of electrons held by the last electron shell of t elements is called the valence electrons.
The number of valence electron in carbon is 4, in Hydrogen is one and in fluorine it is 7. That means, in order to fulfil octet Carbon needs four more electrons, Hydrogen needs one more electron and Fluorine needs one more electron.
Two Hydrogen atom share their only electrons with Carbon. Similarly, carbon shares its two electrons with two Hydrogen atom. Besides, each of the two-fluorine atom share one electron with carbon and partially adopts one electron from Carbon for each.
CH2F2 hybridization
The hybridisation of any compound can e recognised by identifying the overlapping structure created by the participant elements of periodic table. This is the feature that is supportive to the concept of shape of the compounds.
In Difluromethane the hybridisation of centre atom that is carbon has been found to possess Sp3 hybridisation as it gains one electron in its one vacant shell of p orbital which migrates from the s orbital of the element after fulfilling octet state through sharing electrons.
CH2F2 solubility
Solubility of the compounds depends on the internal forces work between the elements. The strong electrostatic force between becomes the barrier in the compounds to make those less soluble in polar solvents.
Difluromethane is Slightly soluble in water. The compound is highly soluble in organic polar solvents such as phenol, methanol etc.
CH2F2 ionic or covalent
The Lewis structure of the compound is quite reliable to denote the characteristic of bonding. As electron share refers to covalent structure and electron transfer refers to ionic, this concept becomes clear with the drawing of Lewis structure of the compounds.
CH2F2 is a covalent compound as here electron sharing takes place instead of complete transfer of the electron from one element to another one.
Read more about Facts on C4H6O3 + H2O
CH2F2 acidic or basic
Acidity and basicity are chemical property with is affected by the electronic transfer or sharing happened in any compound. Lewis gave theory about acidity and basicity that is if a compound gives electron to another atom then it would be considered as Lewis base and the element that would accept the election would be considered as Lewis acid.
The covalent Difluromethane is considered as a neutral organic compound where both the participant elements patricianly donate and accept electrons to make covalent bond.
CH2F2 polar or nonpolar
Polarity is the property that arises due the electronegative difference between the elements participated to form a compound in chemistry.
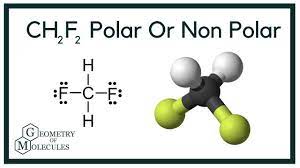
CH2F2 is found as a Polar compound though it has a symmetrical geometry. However, the huge difference between the electronegativity of Carbon and Fluorine make the polarity uplifted in this covalent compound.
CH2F2 tetrahedral
CH2F2 has tetrahedral geometry structure which has already been mentioned above in this article. the tetrahedral structure of compounds imposes that the number of bond pairs is the compound. is four
As CH2F2 has for bond pairs with sigma bonds it is quite clarified that the compound has tetrahedral geometry.
Read more about Facts on C4h6o3 + c7h6o3
CH2F2 linear
If the number of bond pairs present in the compound is found as two then it is recognised that the compound has linear shape in with simplest geometry,.
CH2F2 is a tetrahedral compound which does not possess any linear structure I the series of compounds.
Conclusion
This article has described more than 13 facts about the compound Difluromethane. This compound has been found as an organic compound, which has a particular Lewis structure. That structure is reliable in making conclusions about the chemical and physical properties beholder by the compound in chemistry.
Also Read:
- Hono lewis structure
- Ncl4 lewis structure
- Hcch lewis structure
- Hio4 lewis structure
- Mgso4 lewis structure
- Seo2 lewis structure
- Na3po4 lewis structure
- Mgcl2 lewis structure
- N2o3 lewis structure
- Formaldehyde lewis structure

Hi…..I am Sarnali Mukherjee, a graduate from the University of Calcutta. I love to teach and share knowledge on chemistry. I have gradually gained interest in article writing since one year ago. I would love to acquire more knowledge on my subject in the future.
Let’s connect through LinkedIn: