Clouds and fog are fascinating atmospheric phenomena that have intrigued scientists and students alike for centuries. These visible aggregations of tiny water droplets or ice crystals suspended in the air play a crucial role in our weather patterns, climate, and even technological advancements. In this comprehensive guide, we will delve into the quantifiable data and technical details surrounding clouds and fog, providing a valuable resource for physics students.
Understanding Clouds and Fog: The Fundamentals
Clouds and fog are formed when water vapor in the air condenses into tiny water droplets or ice crystals. The primary difference between clouds and fog is their altitude: clouds are suspended in the sky, while fog is a ground-level cloud. Both, however, are governed by the same physical principles of condensation and the behavior of water in the atmosphere.
The Physics of Condensation
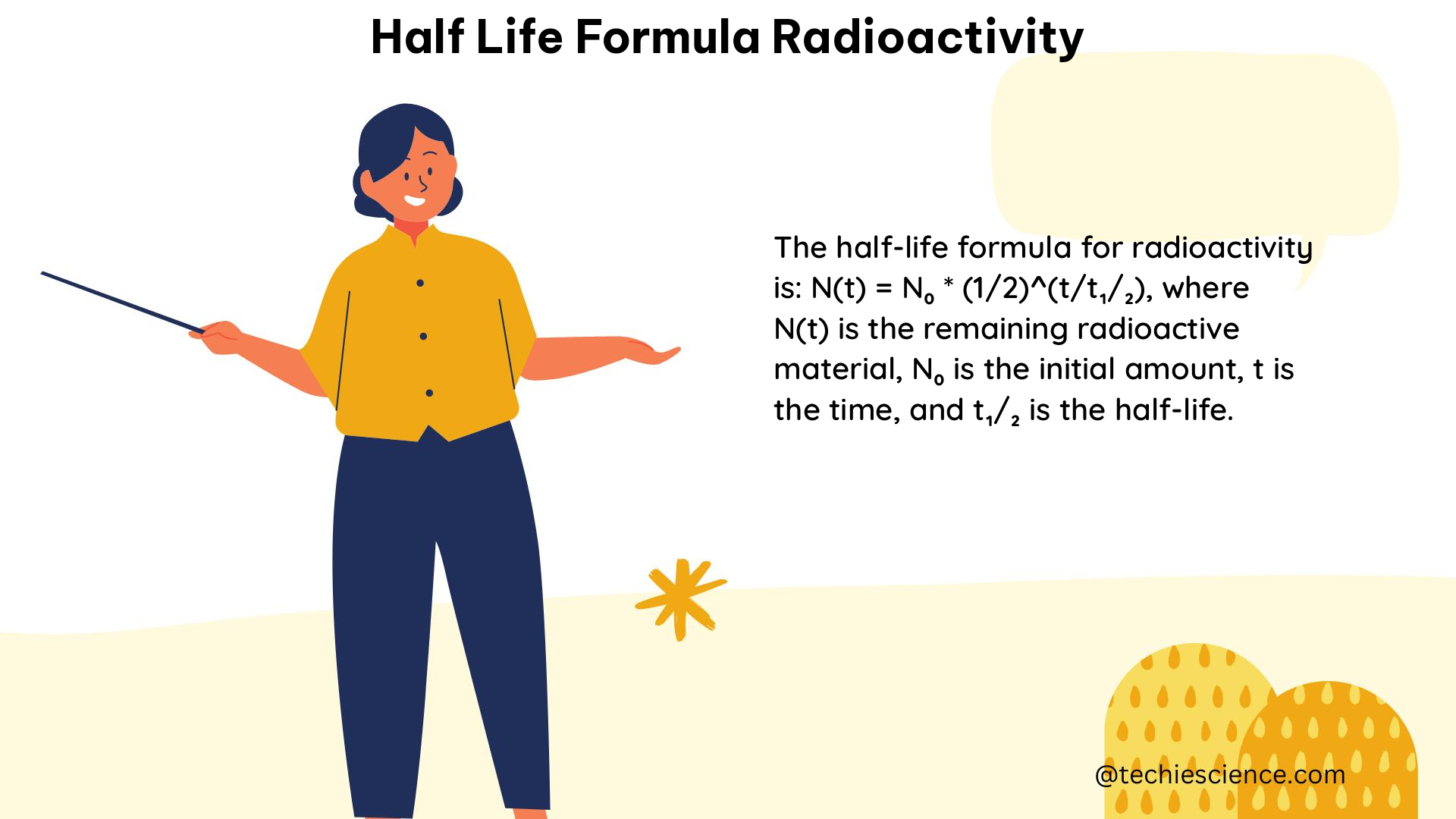
The process of condensation is driven by the relationship between temperature and the saturation vapor pressure of water. As air cools, its ability to hold water vapor decreases, leading to the formation of tiny water droplets or ice crystals. This phenomenon is described by the Clausius-Clapeyron equation, which relates the saturation vapor pressure to temperature:
ln(P_s) = (L/R) * (1/T_0 - 1/T)
where P_s is the saturation vapor pressure, L is the latent heat of vaporization, R is the gas constant, T_0 is the reference temperature, and T is the current temperature.
Cloud and Fog Formation
Clouds and fog form when the air becomes saturated with water vapor, and the excess water condenses onto tiny particles in the atmosphere, known as cloud condensation nuclei (CCN) or fog condensation nuclei (FCN). These nuclei can be composed of various substances, such as dust, smoke, or sea salt, and they provide a surface for the water vapor to condense upon.
The specific conditions that lead to cloud or fog formation can be described using the concept of relative humidity, which is the ratio of the actual water vapor pressure to the saturation vapor pressure at a given temperature. When the relative humidity reaches 100%, the air is said to be saturated, and condensation can occur.
Quantifying Clouds and Fog

To better understand the behavior and impact of clouds and fog, researchers have developed various techniques to quantify their properties. Here are some key data points and measurements related to clouds and fog:
Cloud and Fog Droplet Size Distribution
The size distribution of water droplets or ice crystals within clouds and fog is a crucial parameter that affects their optical properties, precipitation, and interaction with electromagnetic radiation. This distribution can be measured using instruments such as optical particle counters or laser diffraction analyzers. Typical cloud droplet sizes range from 2 to 50 micrometers, while fog droplets are generally smaller, ranging from 1 to 40 micrometers.
Cloud and Fog Liquid Water Content
The liquid water content (LWC) of clouds and fog is a measure of the mass of water per unit volume of air. It is an important parameter for understanding the radiative properties, precipitation processes, and potential impact on aviation and ground-based operations. Typical LWC values for clouds range from 0.01 to 3 grams per cubic meter, while fog LWC is generally lower, ranging from 0.01 to 0.5 grams per cubic meter.
Cloud and Fog Optical Properties
Clouds and fog can significantly affect the transmission of electromagnetic radiation, including visible light, infrared, and microwave wavelengths. The optical properties of clouds and fog are determined by the size, shape, and composition of the water droplets or ice crystals. These properties can be quantified using parameters such as the extinction coefficient, scattering coefficient, and single-scattering albedo. For example, the extinction coefficient of clouds can range from 0.01 to 100 per kilometer, depending on the cloud type and droplet size distribution.
Cloud and Fog Microphysical Processes
The formation, growth, and evolution of clouds and fog involve complex microphysical processes, such as condensation, evaporation, coalescence, and riming. These processes can be studied using advanced instrumentation, such as cloud chambers, wind tunnels, and aircraft-mounted probes. Numerical models, such as those used in weather forecasting, also incorporate detailed microphysical parameterizations to simulate the behavior of clouds and fog.
Satellite and Ground-Based Observations
Advances in remote sensing technology have enabled more comprehensive and quantitative monitoring of clouds and fog. Satellite instruments, such as those on the GOES-R series, can provide detailed information on cloud cover, cloud top height, and cloud optical properties. Ground-based instruments, including lidar, ceilometers, and visibility sensors, can also be used to measure the properties of fog and low-level clouds.
Applications and Implications
The quantifiable data on clouds and fog has far-reaching implications in various fields, from weather forecasting and climate modeling to aviation safety and renewable energy.
Weather Forecasting and Climate Modeling
Accurate representation of clouds and fog is crucial for improving the accuracy of weather forecasts and climate models. The detailed microphysical and optical properties of clouds and fog can be incorporated into numerical weather prediction models to better simulate precipitation, radiation, and other atmospheric processes.
Aviation and Transportation
Clouds and fog can have significant impacts on aviation and ground-based transportation. Reduced visibility due to fog can lead to flight delays, diversions, and increased risk of accidents. Quantifiable data on cloud and fog properties can help develop better visibility sensors, improve landing and takeoff procedures, and enhance decision-making for air traffic control.
Renewable Energy
Clouds and fog can affect the performance of solar and wind energy systems. The optical properties of clouds can influence the amount of solar radiation reaching the Earth’s surface, while fog can impact the operation of wind turbines. Understanding the quantifiable data on clouds and fog can help optimize the design and placement of renewable energy systems.
Environmental Monitoring and Research
Clouds and fog play a crucial role in the Earth’s water cycle and climate system. Quantifiable data on cloud and fog properties can contribute to a better understanding of atmospheric processes, the formation of precipitation, and the interactions between the atmosphere, land, and oceans. This information is valuable for environmental monitoring, climate change research, and the development of more accurate climate models.
Conclusion
Clouds and fog are complex and fascinating atmospheric phenomena that have a significant impact on our daily lives and the environment. By understanding the quantifiable data and technical details surrounding these phenomena, physics students can gain a deeper appreciation for the underlying physical principles and their practical applications. This comprehensive guide has provided a wealth of information on the fundamentals, measurements, and implications of clouds and fog, equipping you with the knowledge to explore these topics further and contribute to the ongoing advancements in atmospheric science and related fields.
References
- Weinman, J. (2015). Cloud vs. Fog: 10 Laws of Fogonomics. [online] LinkedIn. Available at: https://www.linkedin.com/pulse/cloud-vs-fog-10-laws-fogonomics-joe-weinman.
- Gao, S., Zhu, Z., Wang, L., Sweeney, C. and Feng, S. (2017). Estimating the influence of precipitation on changes in atmospheric CO2 concentration. Journal of Atmospheric and Solar-Terrestrial Physics, 154, pp.30-39.
- Wiegner, M., Geiß, A., Mattis, I., Pattantyús-Ábrahám, M. and Bravo-Aranda, J.A. (2021). Characterization of Fog and Low Clouds with Ground-Based Remote Sensing. Atmosphere, 12(6), p.738.
- Ren, Y., Ren, Z., Li, A. and Yan, L. (2017). Fog detection on urban roads through deep learning. Procedia Computer Science, 122, pp.733-740.
- NOAA National Environmental Satellite, Data, and Information Service (NESDIS). (2021). New Satellite Instruments Provide a Step Forward in Detecting Low Clouds. [online] Available at: https://www.nesdis.noaa.gov/news/new-satellite-instruments-provide-step-detecting-low-clouds.