What is Acid Rain?
Acid rain is a kind of precipitation with acidic elements, such as nitric or sulfuric acid, that fall from the atmosphere from types that are dry or wet. This can include rain or any other form such as snow, fog, hail, or even dust that’s acidic.
What Causes Acid Rain?
Acid rain occurs because of sulfur dioxide (SOX) and nitrogen oxide (NOX) are released to the environment because of pollution from various source such as industrialization, chemical reaction and transportation. NOX and SO2 are responsible for producing nitric and sulfuric acids and fall in the ground mix with water and other materials.
As a small section of the SOX and NOX can generated from organic sources like volcanoes, though utmost section of these originates from the burning of fossil fuels actually. The Significant sources of SO2 and NOX from the air would be:
Two-thirds of one and SO2 fourth come from electrical power plants, vehicles, and heavy equipment. The winds can flow SO2 and NOX over the distances may cause acid-rain, which is a severe problem for one and all and not just people lived nearby to that released sources.
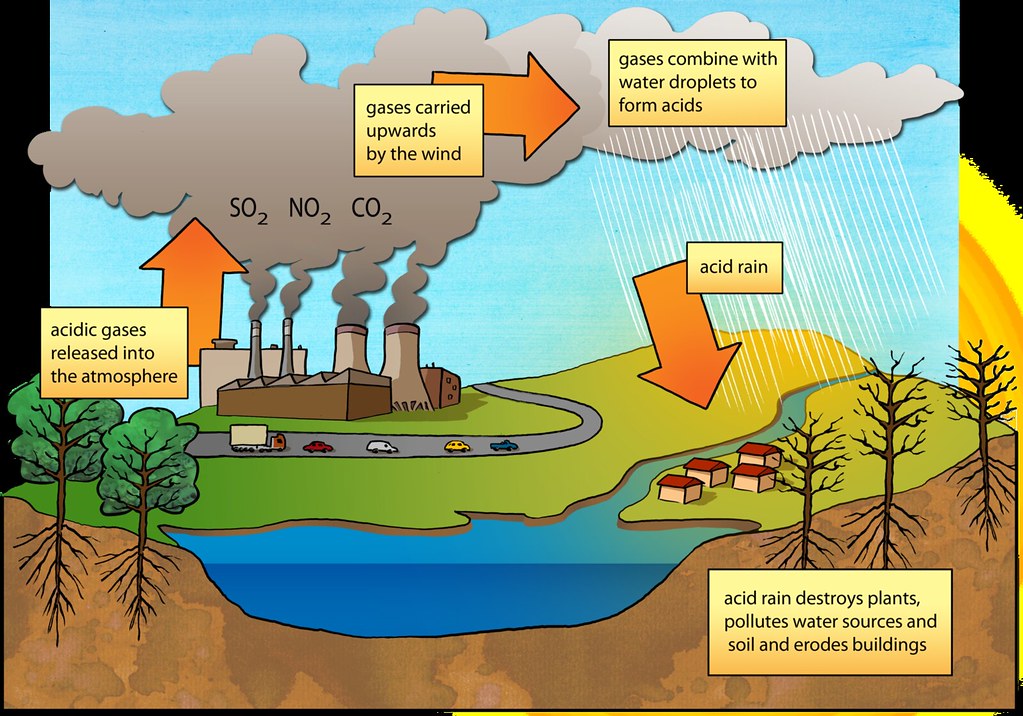
Acid Rain Diagram Image Credit: Siyavula Education and www.flickr.com/
Forms of Acid Deposition
Wet Deposition
It is the most common form of acid rain. The nitric and sulfuric acid are in the atmosphere because of NOX and SOX, may easily drop to the earth surface and mixed with rain-water, may form snow/ ice, or drop as fog, or hail too. These types of deposition are termed as wet deposition and most commonly observed acid rain in the atmosphere.
Dry Deposition
Gases and particles may deposit as dry residue in the air in the lack of moisture. The acidic pollutants and particles can deposit to surfaces (water bodies, plants, plants ) fast or respond during atmospheric transport to produce particles that could be detrimental to human health. This water flows over and through the floor, and may damage wildlife and plants, like fish and insects when the acids have been washed off a surface from the rain.
The total quantity of acidity in the air that residue to earth through dry deposition is dependent upon the number of rainfall an area receives. In desert regions, By way of instance, the proportion of deposition is greater than an area that receives inches of rain.
Measuring Acid Rain
The parameter like ‘acidity’ and ‘alkalinity’ are the standard quantities to be measured for acid rain by means of a pH level, for normal water it in 7.0 acts as neutral water
More acidic if pH < than 7 and if pH> 7) then it becomes more alkaline. The usually found acid rain have pH measures in-between 4.2 and 4.4 range.

Effects of Acid Rain
Impacts of Acid Rain on Plants and Trees
Acid rain is also leaching aluminium in the ground material. That aluminium is bad for animal as well as plant too. Acid rain eradicates minerals and nutrients that plants prerequisite to nurture better. Dead or dying trees by acid rain is found commonly because of this reason. The trees become incapable to engross sunlight, makes them weaker and to resist cold environments.
Episodic Acidification
In episodic acidification, melting snow and rain downpours is resultant. It can temporarily undergo acid rain consequences; the soil can’t buffer it, and once the snow or downpour brings quantities of deposition. The short duration of higher acidity (i.e., lower pH) may lead to short-term stress in the ecosystem.
Nitrogen Pollution
The direct impacts merely the problem of rain acidity, Nitrogen is also contained in acid rain, and this can affect ecosystems. The nitrogen pollution in our coastal waters is accountable for shellfish populations and decreasing fish in certain areas.
Buffering Capacity

Many forests, streams, and lakes that encounter acid rain do not suffer consequences by neutralizing the acidity at the rainwater flowing through it. The soil in these regions can buffer the acidity. This ability is dependent upon the depth and composition of the soil and also bedrock under its kind. In regions like mountainous areas, the soils lack the ability to sufficiently counteract the acid in the rainwater. Because of this, these regions are especially vulnerable, and aluminium and the acid may collect in lakes, streams, or the soil.
Also Read:
- Boiling point of glacial acetic acid
- Boiling point of benzoic acid
- Is adenine an amino acid
- Boiling point of formic acid
- Boiling point of ethanoic acid
- Boiling point of sulphuric acid
- Is hbr acid
- Boiling point of acetic acid

I am Subrata, Ph.D. in Engineering, more specifically interested in Nuclear and Energy science related domains. I have multi-domain experience starting from Service Engineer for electronics drives and micro-controller to specialized R&D work. I have worked on various projects, including nuclear fission, fusion to solar photovoltaics, heater design, and other projects. I have a keen interest in the science domain, energy, electronics and instrumentation, and industrial automation, primarily because of the wide range of stimulating problems inherited to this field, and every day it’s changing with industrial demand. Our aim here is to exemplify these unconventional, complex science subjects in an easy and understandable to the point manner.
