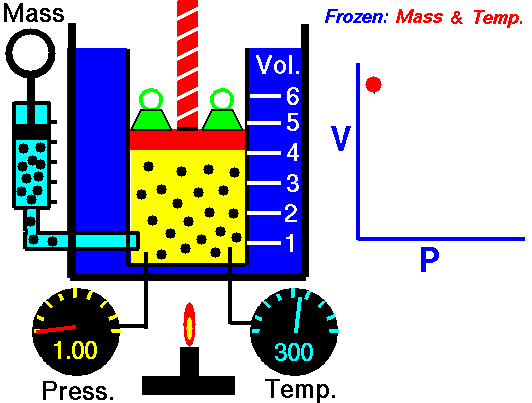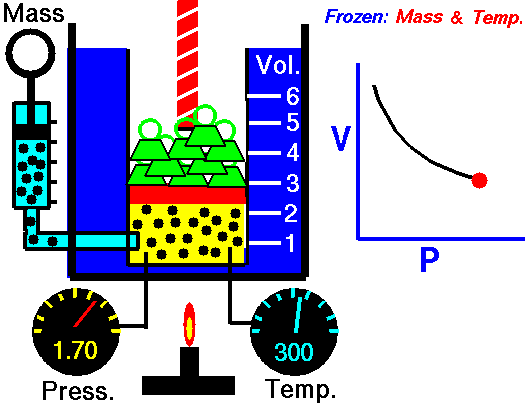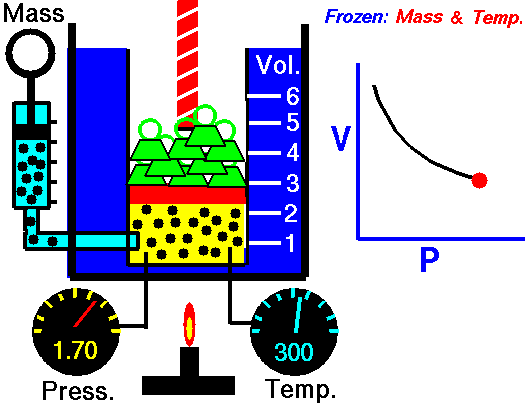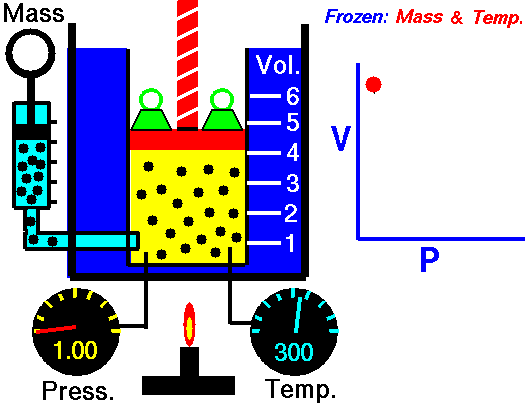
In this article “Thermal expansion example” with detailed explanations will be discuss. Thermal expansion example is use for a substance when it is expands due to high temperature or low temperature.
9+ Thermal Expansion Example is listed below,
- Thermometers
- Removing tight lids
- Bimetallic strip
- Riveting
- Thermostats
- Cracks appearing in the roads
- Expansion joints
- Metal frame windows need rubber
- Tyre of the vehicle gets warm
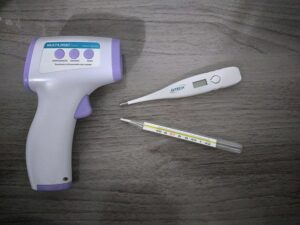
Thermometers:-
Thermometer is the example of thermal expansion. Thermometer helps us to measure the amount of temperature or temperature gradient of a system. The thermometer used in many purpose such as science research, manufacturing field or practice of medicine, in automotive sector and many more. Thermometer is a tube which is made of glass material when thermometer is placed in a hot substance the inside liquid takes temperature and increases its volume. In the thermometer body equally divided the measurement scale. When the inside liquid rises up easily we can understand the temperature of the substance.
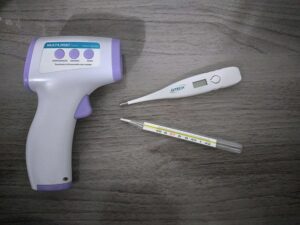
Image Credit – Wikimedia Commons
Removing tight lids:-
Sometime a lid of a jar too tight for this difficulty can face for open the lid. We all are know that when expansion is produce in a substance that time the substance is increases its volume area and length. So, facing difficulty opening of a lid if heat up then it’s expand its area and easy to open.

Image Credit – snappygoat.com
Bimetallic strip:-
Bimetallic strip is a device which is convert temperature into mechanical displacement. Bimetallic strip contain two different type of metal and work on the thermal expansion principle. In the bimetallic strip two metals are expand in different temperature difference.
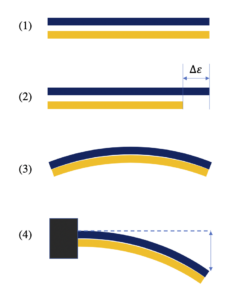
Image Credit – Wikimedia Commons
Types:
Bimetallic strips classified in two sections,
Spiral strip type:
Spiral strip type bimetallic strips is contains a structure of spiral like in it a pointer is added thus the pointer can measure the temperature. When the structure of the spring shaped is heated the metals shown their property of thermal expansion and the structure of the spring shaped is deform when temperature is fall. In this period the scale is recorded the amount of temperature. Normally by the help of spiral strip type bimetallic strips we can record the temperature of ambient.
Helical type:
Helical type bimetallic strips is contains a structure of helical like in it a pointer is added thus the pointer can measure the temperature. When the structure of the helical shaped is heated the metals shown their property of thermal expansion and the contracts on cooling. In this period the scale is recorded the amount of temperature. Normally by the help of helical strip type bimetallic strips we can record the temperature of industrial applications.
Advantages of Bimetallic strips:
- Less cost
- Robust
- Simple in use
- External power not needed
- Give accuracy in between ± 2 to 5
Disadvantages of Bimetallic strips:
- Low temperature does not work properly.
- Measure up to 4000 centigrade
Application:
- Bimetallic strip use in a fire detector or fire alarm
- Bimetallic strip use in mechanical clocks for minimize the errors during the changes of temperature.
- Heaters
- Iron box
- Heat engine
- Thermistor
- Fans
Riveting:-
Riveting is work on the basic of thermal expansion. Riveting is a fixed mechanical fastener. Rivets made with aluminium or steel and used to joining the metal pieces. River joints contain gun, rivet pin and operation with rivet is known as riveting.

Image Credit – Wikimedia Commons
Types:
- Button head rivet
- Join our Newsletter rivet
- Belt rivet
- Hollow rivet
- Boiler construction rivet Explosive rivet
Thermostats:-
Thermostat is work on the basic of thermal expansion. Thermostat is a machine by which we can detect the amount of temperature changes. The thermostat uses in valves, switches, relays and many more.
Thermostat is a device which is works on two purposes one is for measurement and another one is for controlling function.

Image Credit – snappygoat.com
Cracks appearing in the roads:-
Cracks appearing in the roads it are another example of thermal expansion. Cracks in the road mainly happened in the hot afternoon when the temperature is rises too up. In the surrounding the temperature is rise up above the 90 degree and wrap and buckle is appearing.

Expansion joints:-
An expansion joint is an example of bellows type device. Expansion joints are mainly used for absorbing thermal expansion. In this way the expansion joints are make thus it could grip and holds the parts together when safely taking temperature induced expansion, vibration and also construction of a building material.
Expansion joints are used in railway tracks, sidewalks, buildings, ships, piping system, bridges.

Image Credit – Wikimedia Commons
Metal frame windows need rubber:-
Now in builds or other construction windows are made of metal. In these windows thermal expansion is happened for this particular reason rubber is use outside the frame of the window. Rubber is not good for heat conductor for this reason thermal expansion can be prevents and the frame will be stay at its correct position without any damage.
Tyre of the vehicle gets warm:-
When a vehicle is run for a long distance that time the tyre of the vehicles getting warm and thermal expansion is appear. Heat plays a vital role for getting warm of the vehicle tyres. When heat is increases the inside pressure is also increases for this reason the temperature is also goes up.
Frequent asked questions:-
Question: – Explain the coefficient of thermal expansion for matter.
Solution: – Thermal expansion coefficient is actually a physical condition of substance to change its area, shape, density and volume under changing of temperature. Thermal expansion is not including the phase transitions. The S.I unit of the thermal expansion is per Kelvin.
The equation for thermal expansion coefficient is,
Where,
∝ = Coefficient of thermal expansion for gaseous matter
V = Volume for gaseous matter
T = Temperature for gaseous matter
P = Pressure for gaseous matter
For particularly 1 mole ideal gaseous substance PV = RT,
Where,
∝ = R/PV = 1/T
Types of thermal expansion coefficient:
The thermal expansion coefficient can be divided in three sections,
- Linear expansion coefficient
- Area expansion coefficient
- Volume expansion coefficient
Linear expansion coefficient:-
Linear expansion coefficient can be explained as, changing of length due to temperature.
Linear expansion coefficient can be written as,
Where,
ΔL= Changing in length
L0= Original length
∝ = Length expansion coefficient
L = Expanded length
Δ T = Temperature difference
Area expansion coefficient:-
Area expansion coefficient can be explained as, changing of area due to temperature.
Area expansion coefficient can be written as,
Where,
ΔA= Changing in area
A0= Original area
∝= Area expansion coefficient
A = Expanded area
Δ T= Temperature difference
Volume expansion coefficient:-
Volume expansion coefficient can be explained as, changing of volume due to temperature.
Volume expansion coefficient can be written as,
Where,
Δ V = Changing in volume
V0 = Original volume
∝ = Volume expansion coefficient
V = Expanded volume
ΔT= Temperature difference
Question: –
Rup daily uses a rod or his gardening purpose, one day he forgets to bring the rod at his own house. The length of the rod is 10 meter at the temperature of 39 degree centigrade. After forget the rod the length of the rod became 15 meter and that time the temperature is 35 degree centigrade.
Now determine the thermal expansion coefficient for the rod.
Solution: – Given data are,
Changing in length ΔL = (15 – 10) meter = 5 meter
Original length L0 = 10 meter
Length expansion coefficient ∝= ?
Expanded length L = 15 meter
Temperature difference Δ T = (39 – 35) degree centigrade = 4 degree centigrade
Absolute temperature = T = (273 + 4) K = 278 K
Rup daily uses a rod or his gardening purpose, one day he forgets to bring the rod at his own house. The length of the rod is 10 meter at the temperature of 39 degree centigrade. After forget the rod the length of the rod became 15 meter and that time the temperature is 35 degree centigrade.
The thermal expansion coefficient for the rod is 17 x 10 -4 K -1