In this article we are going to analyze the SRO Lewis structure and various facts about it.
Strontium oxide is produced when strontium reacts with oxygen. When strontium is burned in presence of air results in a mixture of strontium oxide and strontium nitride.
SRO Lewis Structure
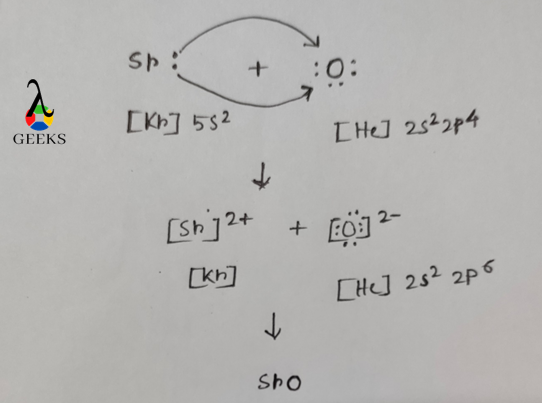
SRO is formed by two elements i.e. one is strontium and other is oxygen. Strontium has atomic number 38. Its electronic configuration is [Kr]5s2. When it losses two electron from 5s orbital it gets the nearest noble gas configuration i.e. Kr(Z=36) which is a stable electronic configuration because in the valence shell of Kr octet is fulfilled.
When Strontium loss 2 electrons by the above process Sr+2 is formed. In case of oxygen, it has atomic number 8. Its electronic configuration is [He]2s22p4. When it gains 2 electrons which is rejected by Strontium it gets nearest noble gas configuration i.e. Ne(Z=10) which is a stable electronic configuration. O2- ion has octet fulfilled valance shell electronic configuration.
Lewis Structure Diagram of SRO
SRO Lewis Structure Formal Charges
In Strontium Oxide overall formal charge on the compound Is Zero(0).When SRO ionizes Sr+2 and O2- ion is formed. Strontium +2 charge is neutralized by Oxygen -2 charge. In the crystal lattice structure of strontium oxide equal number of Sr+2 and O-2 is present so that overall formal charge is Zero.
SRO Lewis Structure Lone Pair
In the Lewis structure of Strontium Oxide zero lone pair present on strontium and two lone pair present on oxygen atom. This is due to the fact that Strontium has lost its two electron i.e. one lone pair and converted to Sr+2 which has no lone pair on it. But in case of Oxygen atom it accepts two electrons from strontium and converted into O2- which has two lone pair on it.
SRO Hybridization
In strontium Oxide Sr+2 and O2- ion is present. Strontium oxide exists in a cubic crystal lattice structure. Both strontium and oxygen have d2sp3 hybridization i.e. octahedral co-ordination geometry. In the lattice structure of strontium oxide each Sr+2 ion is surrounded by six oxygen atoms and each O2- ion is surrounded by six Sr+2 ion. Hence both strontium ion and oxide ion have co-ordination number equals to 6.
SRO Lewis structure Resonance
Strontium Oxide is overall a neutral molecule with zero formal charge on it. But during resonance takes place strontium oxide breaks into Sr+2 ion and O2- ion. After resonance both strontium and oxide ion are stabilized because both stable nearest noble gas configuration i.e. Strontium ion acquires krypton(Kr) noble electronic configuration and Oxide ion acquires Neon(Ne) noble gas configuration.
SRO Lewis Structure Octet Rule
In strontium Oxide both Strontium and Oxygen have their octet fulfilled. During the formation of Strontium oxide Strontium losses two electron to get nearest noble gas configuration i.e. (Kr) which have 8 electrons in its valance shell (4s24p6) and Oxygen accepts two electrons from strontium to get nearest noble gas configuration i.e. (Ne) which also have 8 electrons in their valance shell (2s22p6). Hence both atoms get their octet fulfilled.
SRO Polar Or Nonpolar
Strontium Oxide is a polar compound. This is because in strontium oxide both strontium and oxygen have different electronegativity and hence opposite dipole creates on strontium and oxygen. Due to less electronegativity of strontium it acquires positive dipole on it and due to more electronegativity of Oxygen atom it gets negative dipole. Due to this opposite dipole that exists in strontium Oxide, it is an ionic compound as well as polar compound.
SRO Uses
Strontium Oxide has various useful applications. It is largely used in cathode ray tubes where strontium is present by 8% of its weight. Now a days Strontium oxide used for making television picture tube glasses. It is also used in glass, optic, and ceramic industry. In recent times for the preparation of strontium SRO is used as a starting material which is being heated with aluminum under vacuum. It is the best method for the production of pure strontium. It also has application in medical industry.
Detailed Fact about SRO
Strontium Oxide is an ionic compound. In the Lewis structure both strontium and oxygen have fulfilled their octet by the process of resonance in which strontium losses two electron and oxygen accepts two electrons. This is because due to less electronegativity strontium gains positive charge and oxygen gains two electrons due to higher electronegativity.
SRO exists in cubic crystal lattice structure in which both Strontium and Oxygen have octahedral arrangement around each other. SRO is a polar molecule due to electronegativity difference between Strontium and Oxygen atom.
Frequently Asked Question about SRO(FAQ)
What happens when Strontium Oxide Reacts with Water?
Strontium Oxide reacts with Water vigorously to form strontium hydroxideas white precipitate with the evolution of heat.
SRO + H20 = SR(OH)2
Why SRO is soluble in Water?
As strontium Oxide is a polar compound, it dissolves in polar solvent as for example water.
Also Read:
- Icl5 lewis structure
- Hio4 lewis structure
- Brf4 lewis structure 2
- Snf3 lewis structure
- Cs lewis structure
- Bro3 lewis structure
- Snf2 lewis structure
- Hclo2 lewis structure
- Li2o lewis structure
- If7 lewis structure

Hi….I am Susanta Maity. I have completed my Masters from Vidyasagar university with a specialization in organic chemistry.
I love to write complicated Chemistry concepts in understandable and simple words.
Let’s connect through LinkedIn: